The Bohr Model of the Atom
Brief Description
A model proposed by Niels Bohr to support his hypothesis about electrons in a hydrogen atom. This is a model of the atomic structure in which electrons travel around the nucleus in well-defined orbits determined by quantum conditions. A transition from a higher orbit to a lower orbit will release quantized energies of light, which would explain the light spectrum emitted by an element.Definitions
Model:A tentative description of a system or theory that accounts for all of its known properties.
Atom:
The smallest particle of an element, indivisible by chemical reaction, that can either exist alone or enter into chemical combination. An atom consists of a central positively charged nucleus surrounded by a sufficient number of negatively charged electrons to balance the nucleus.
Emission Spectrum:
The spectrum of frequencies of radiation (light) emitted by an atom or a molecule in a transition from a higher state to a lower energy state.
Electronic state:
A condition or mode of being with regard to a set of circumstances; position.
Bohr, Niels:
The Danish physicist that attempted to account for the emission spectrum of atomic hydrogen by proposing that the single electron in the hydrogen atom travels in definite circular orbits around the central nucleus and that only certain orbits were possible at certain energies. This is what we call the Bohr model.
What it is:
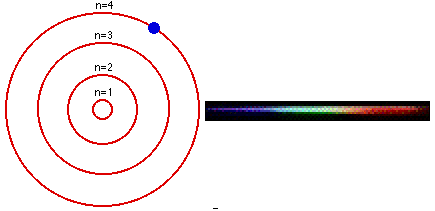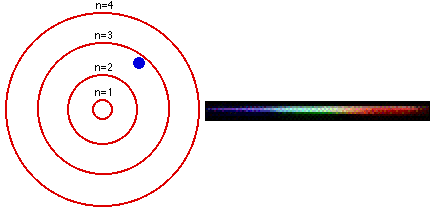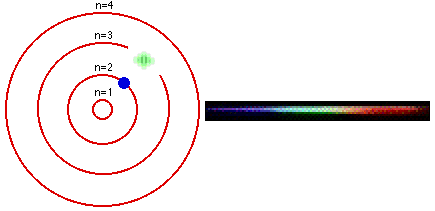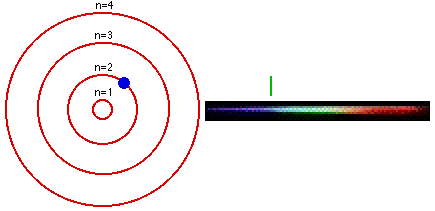
As we all know, from our chemistry book, each element in the periodic table has its own characteristic emission spectrum. The spectrum is a result of applying some kind of energy, such as heat, to a sample and passing the light emitted through a slit, to obtain a narrow beam. Then the beam is sent through a glass prism to disperse the light into the spectrum. Each line in the spectrum corresponds to an electronic transition between energy levels associated with the element. These levels are called energy states.The lowest energy state an atom can be at is called its ground state. When an electron in an atom has absorbed energy it is said to be in an excited state. An excited atom is unstable and tends to rearrange itself to return to its lowest energy state. When this happens, the electrons lose some or all of the excess energy by emitting light. Light is only emitted at certain frequencies, each corresponding to a particular electronic transition within the allowed states.
The Bohr model is used to account for the spectrum of the hydrogen atom, but the basic idea is the same for all elements. The single electron in hydrogen revolves around the nucleus in one of a limited number of circular orbits. When it is in the orbit closest to the nucleus it is in its ground state, this electron is in the valence band of the element.
When hydrogen is heated, or some other energy is being applied to it, the atom absorbs the energy and the electron becomes excited and "jumps" to an orbit farther from the nucleus. In other words, it goes up to a higher energy state. The more energy is applied to the atom, the higher in states the electron goes until the point where it becomes a free electron and no longer part of the atom.
The lines in an emission spectrum occur when the electron loses energy, "falls back", from a higher energy state to a lower one emitting photons at different frequencies for different energy transitions.
You might want to take a look at the movie for a more visual explanation.
Another movie you might want to look at would be the demo for a silicon atom. If you're still curious, you can try another website that goes more in detail: Review Chemistry