You must login before you can run this tool.
Analyzing Correlation Structure in a Model of Neural Activity-Dependent Homeostatic Plasticity (ADHP)
Users can manipulate the parameters of an abstract model of ADHP and analyze the correlation structures that arise (or don't arise) as a result.
Category
Published on
Abstract
Background/Intuition:
It has been observed in various model systems that neurons which are chronically hypo- or hyper-active adjust their expression of various ion channels to restore target levels of excitation. This process is thought to be dependent on intracellular Calcium concentration, which tracks activity level. As such, it is called activity-dependent homeostatic plasticity (ADHP). Putting aside the nonlinear properties of some ion channels which allow for complex neural behaviors like bursting, it makes logical sense that achieving such robustness would necessitate the following homeostatic rules:
Hyper-active neurons should downregulate their inward/excitatory conductances and upregulate their outward/inhibitory conductances. Hypo-active neurons should upregulate their inward/excitatory conductances and downregulate their outward/inhibitory ones.
However, abstract models of ADHP proposed by O'Leary et al. (2013) have shown that other, more counterintuitive sets of rules can also confer robustness to certain perturbations. Moreover, it is proposed that the particular set of rules determines pairwise correlations that emerge among the ion channel densities of the restored neurons.
More specifically, there exist many combinations of ionic conductances which would allow a neuron to achieve its desired equilibrium calcium concentration and activity level. (This property is known as parameter space degeneracy.) In one case analyzed by the paper, these solutions lie on a plane in 3D conductance space (pink plane below). Among these possible solutions, only a subset are realized by any one implementation of ADHP. The authors hypothesize that this subset exhibits a specific correlation structure which is determined by the set of rules guiding ADHP.
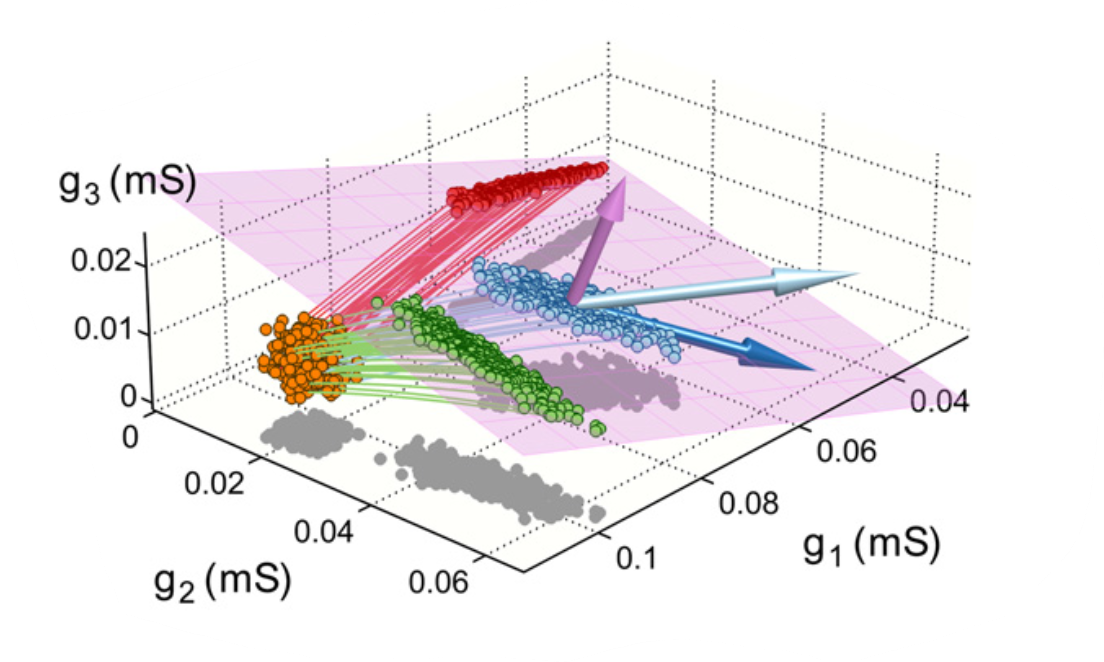
(Adapted from O'Leary et al., 2013, Figure 1D)
We suspect, however, that these correlation structures could more readily be explained by the limited sample of initial conditions in this study. The rules employed by ADHP dictate a general direction of motion in parameter space, and when applied to this small set of initial points, several distinct clusters of equilibrium solutions emerge depending on which direction is chosen. But, we aim to show, these clusters would not emerge if initial conditions were more widely sampled through the space around the plane. In this case, end points will be just as widely spread over the plane of possible solutions. Thus, it is not the homeostatic tuning rules which govern the correlation structure of restored neurons, but only the implicit degeneracy of the parameter space.
List of Modules:
- Users can examine the effects of changing homeostatic rules on the trajectory of a single neuron through parameter space
- Change the center and spread of the sample of initial conditions and visually examine the set of equilibrium solutions that arise under different sets of homeostatic rules
- Quantitatively analyze the correlation structures among the set of equilibrium solutions produced in (2)
Main Inspiration Paper:
O’Leary, T., Williams, A. H., Caplan, J. S., & Marder, E. (2013). Correlations in ion channel expression emerge from homeostatic tuning rules. Proceedings of the National Academy of Sciences, 110(28). https://doi.org/10.1073/pnas.1309966110
Other Related Papers:
Caplan, J. S., Williams, A. H., & Marder, E. (2014). Many parameter sets in a multicompartment model oscillator are robust to temperature perturbations. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(14), 4963–4975. https://doi.org/10.1523/JNEUROSCI.0280-14.2014
O’Leary, T., Williams, A. H., Franci, A., & Marder, E. (2014). Cell Types, Network Homeostasis, and Pathological Compensation from a Biologically Plausible Ion Channel Expression Model. Neuron, 82(4), 809–821. https://doi.org/10.1016/j.neuron.2014.04.002
Santin, J. M., & Schulz, D. J. (2019). Membrane Voltage Is a Direct Feedback Signal That Influences Correlated Ion Channel Expression in Neurons. Current Biology, 29(10), 1683-1688.e2. Scopus. https://doi.org/10.1016/j.cub.2019.04.008
Schulz, D. J., Goaillard, J.-M., & Marder, E. (2006). Variable channel expression in identified single and electrically coupled neurons in different animals. Nature Neuroscience, 9(3), Article 3. https://doi.org/10.1038/nn1639
Temporal, S., Desai, M., Khorkova, O., Varghese, G., Dai, A., Schulz, D. J., & Golowasch, J. (2012). Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. Journal of Neurophysiology, 107(2), 718–727. https://doi.org/10.1152/jn.00622.2011
Temporal, S., Lett, K. M., & Schulz, D. J. (2014). Activity-dependent feedback regulates correlated ion channel mRNA levels in single identified motor neurons. Current Biology: CB, 24(16), 1899–1904. https://doi.org/10.1016/j.cub.2014.06.067
Powered by
Plotly 5.5.0, numpy, sklearn
Cite this work
Researchers should cite this work as follows:


