Electrochemical Potentiometric Sensors for Environmental Monitoring
Electrochemical Potentiometric Sensors for Environmental Monitoring
-
 1. Electrochemical potentiometric…
0
00:00/00:00
1. Electrochemical potentiometric…
0
00:00/00:00 -
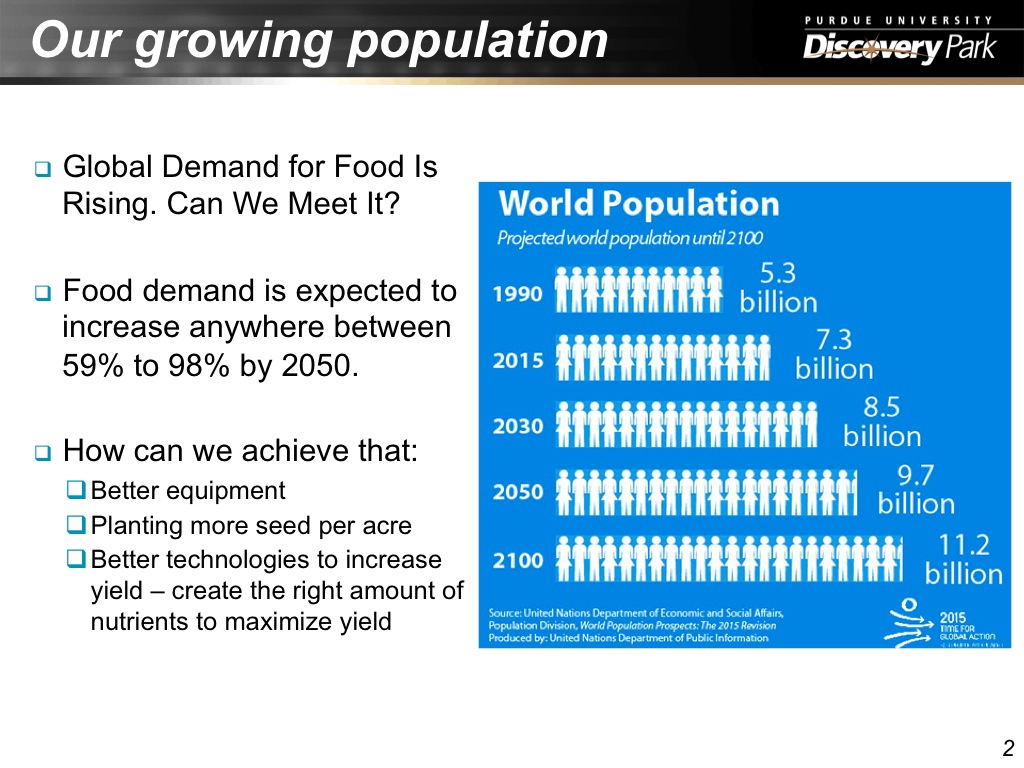 2. Our growing population
32.2655989322656
00:00/00:00
2. Our growing population
32.2655989322656
00:00/00:00 -
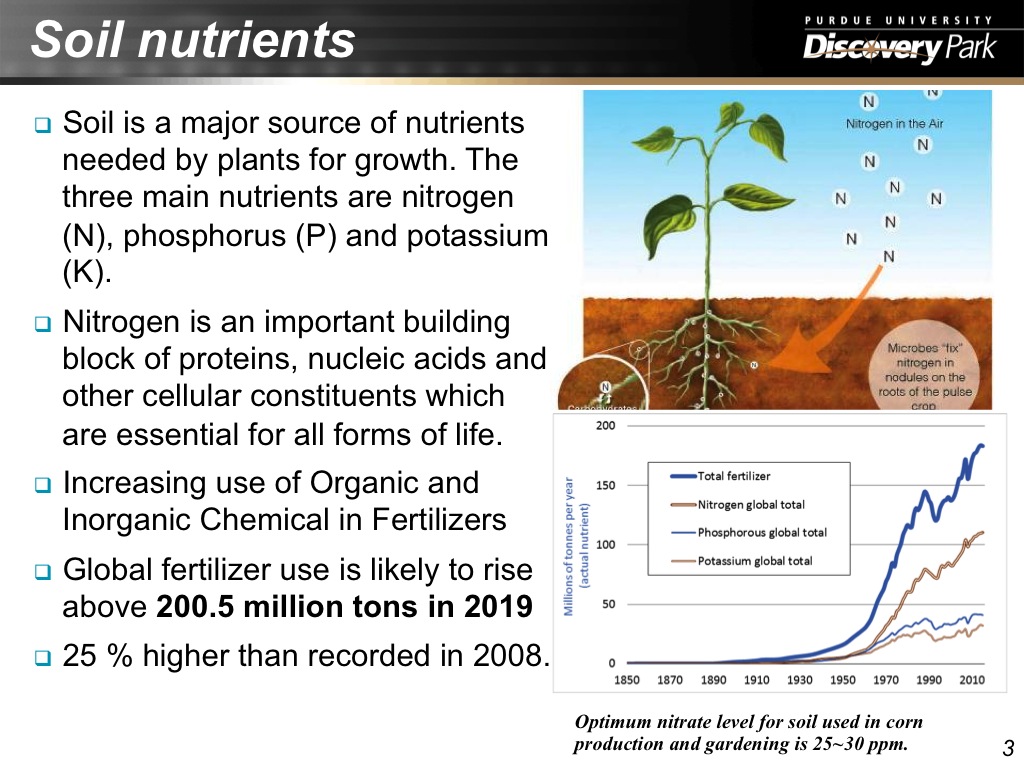 3. Soil nutrients
117.51751751751752
00:00/00:00
3. Soil nutrients
117.51751751751752
00:00/00:00 -
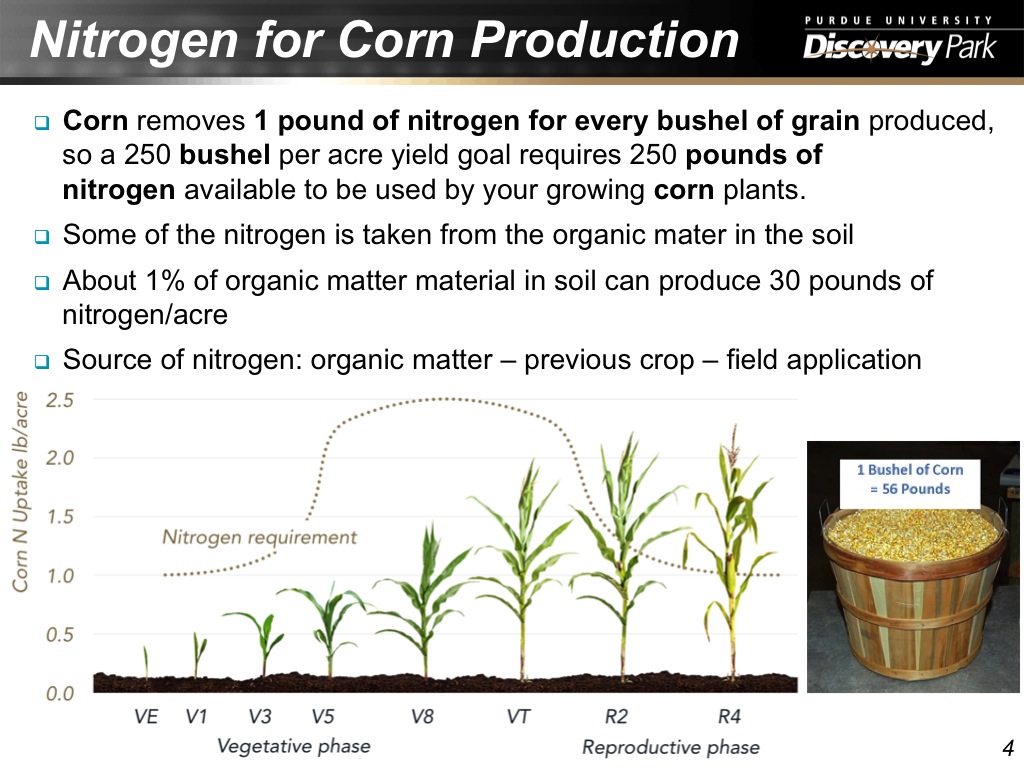 4. Nitrogen for Corn Production
185.58558558558559
00:00/00:00
4. Nitrogen for Corn Production
185.58558558558559
00:00/00:00 -
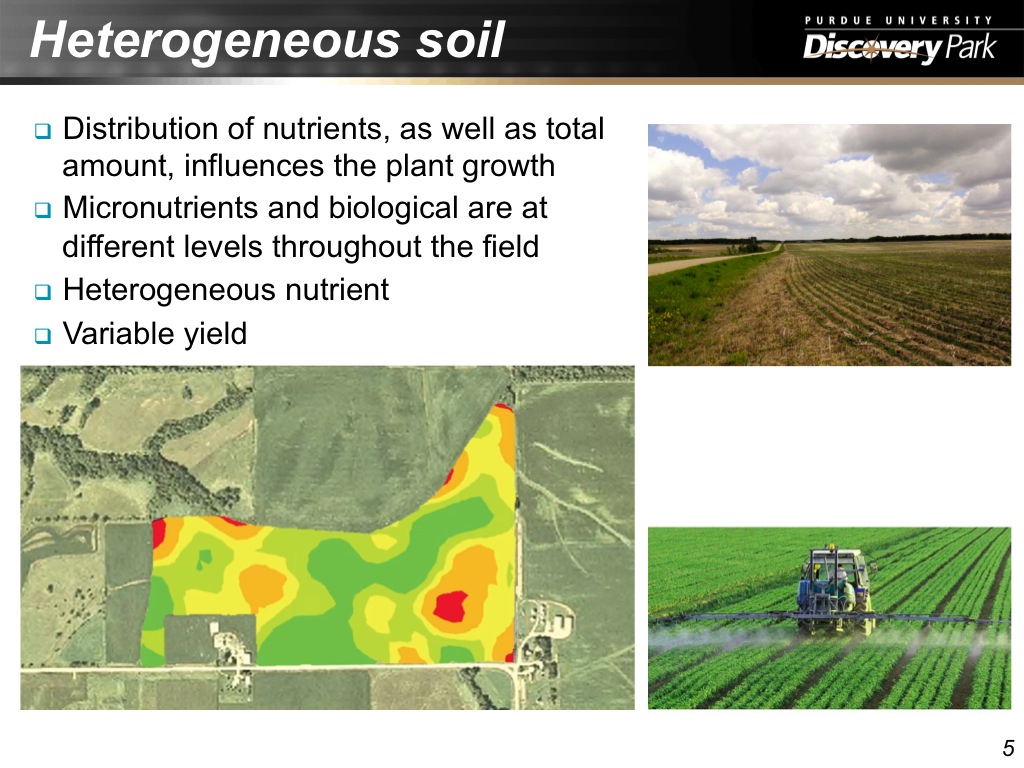 5. Heterogeneous soil
231.53153153153153
00:00/00:00
5. Heterogeneous soil
231.53153153153153
00:00/00:00 -
 6. Overusing of Fertilizers
274.60794127460792
00:00/00:00
6. Overusing of Fertilizers
274.60794127460792
00:00/00:00 -
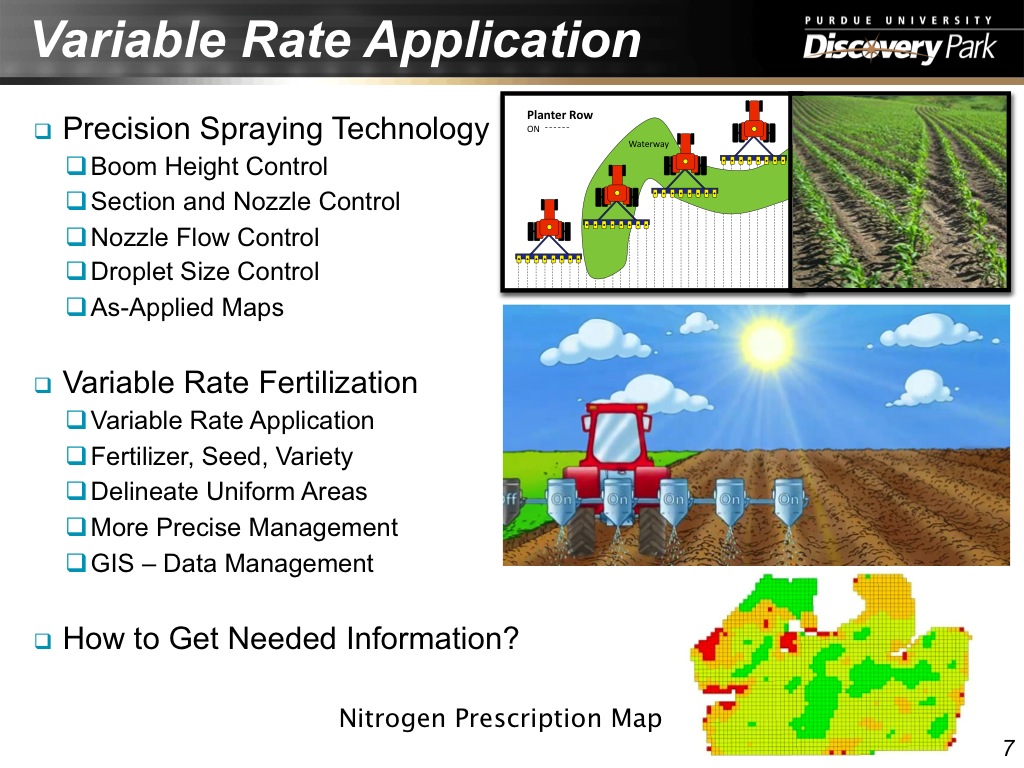 7. Variable Rate Application
310.1101101101101
00:00/00:00
7. Variable Rate Application
310.1101101101101
00:00/00:00 -
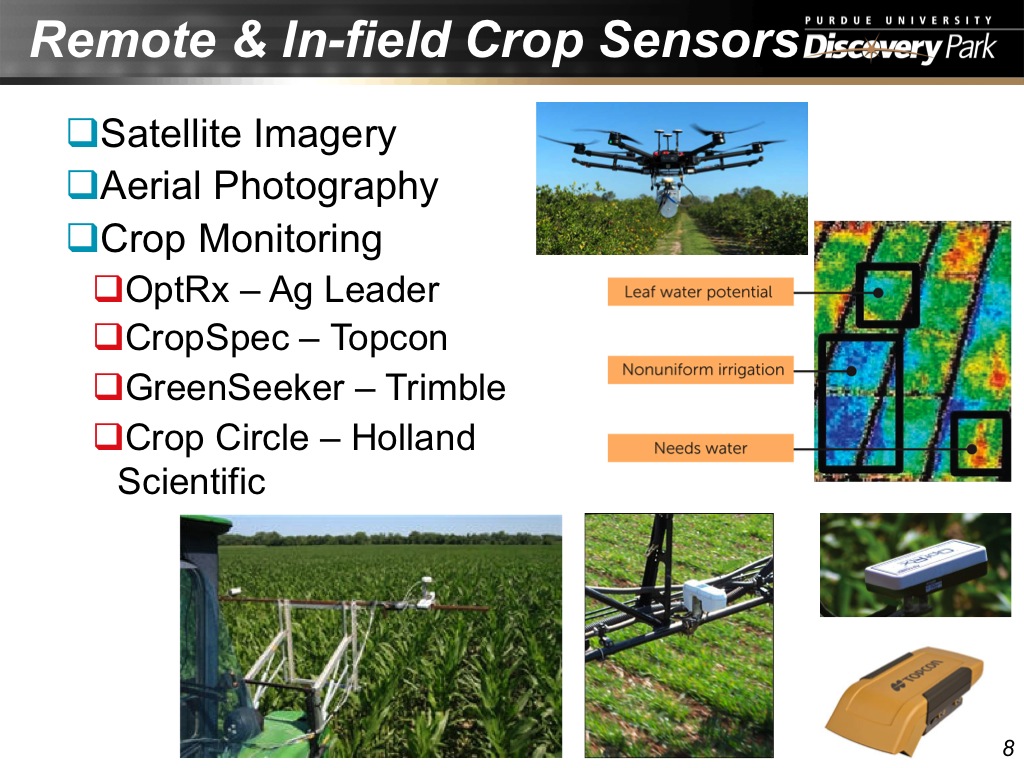 8. Remote & In-field Crop Sensors
346.68001334668
00:00/00:00
8. Remote & In-field Crop Sensors
346.68001334668
00:00/00:00 -
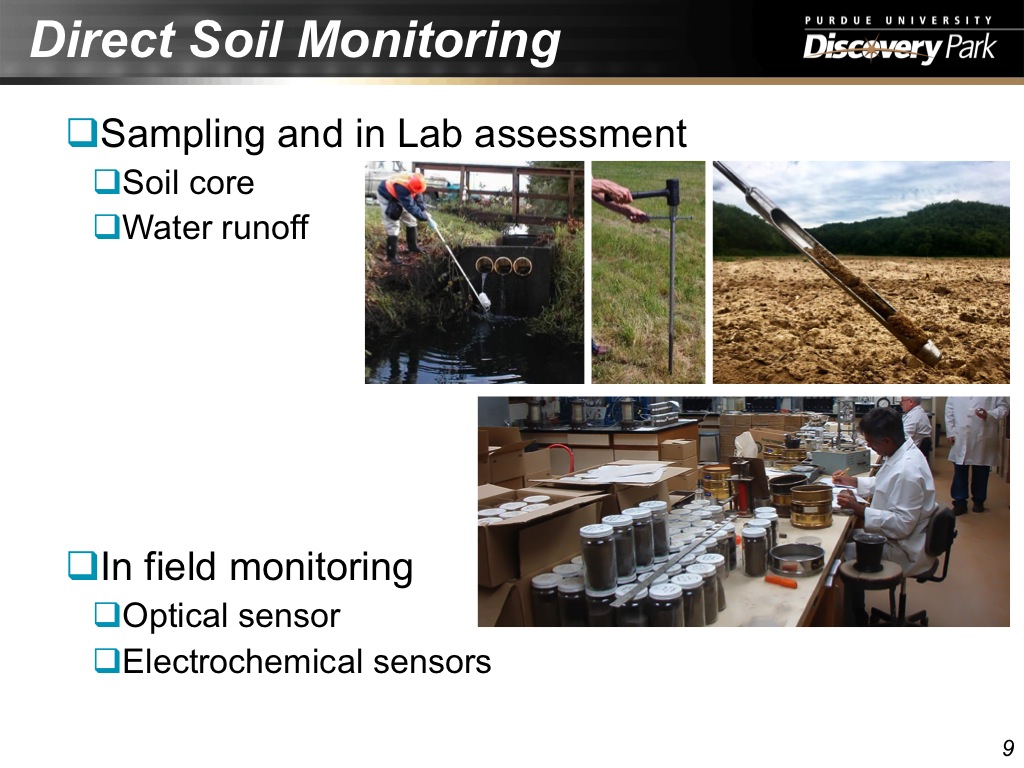 9. Direct Soil Monitoring
420.78745412078746
00:00/00:00
9. Direct Soil Monitoring
420.78745412078746
00:00/00:00 -
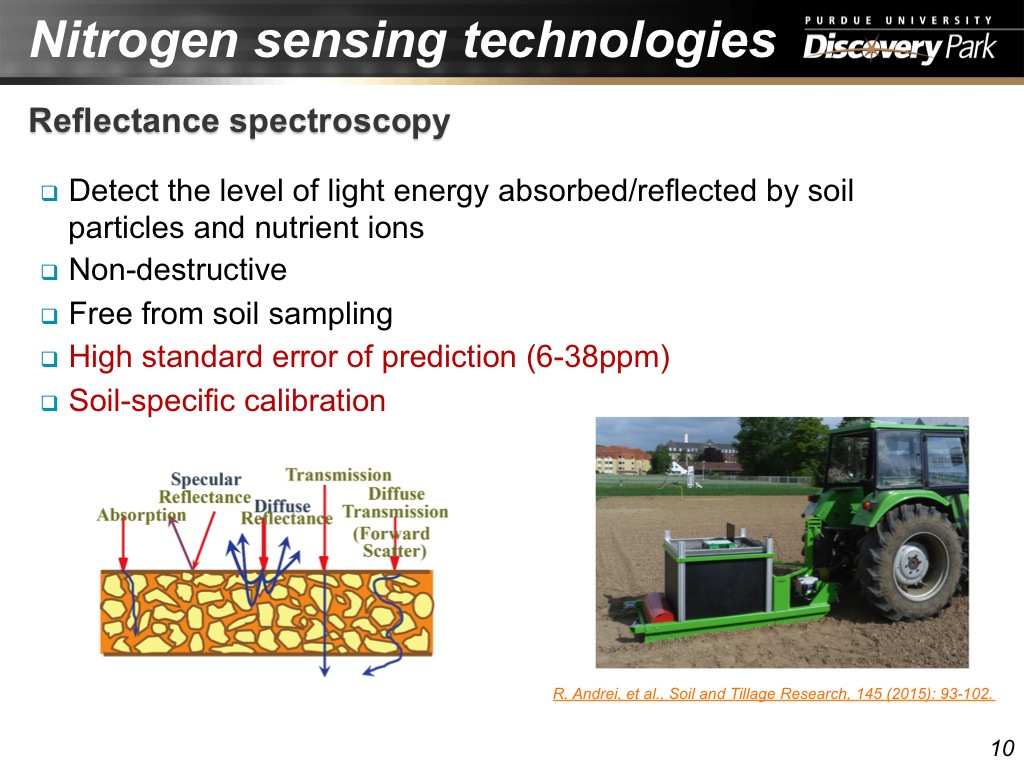 10. Nitrogen sensing technologies
488.78878878878879
00:00/00:00
10. Nitrogen sensing technologies
488.78878878878879
00:00/00:00 -
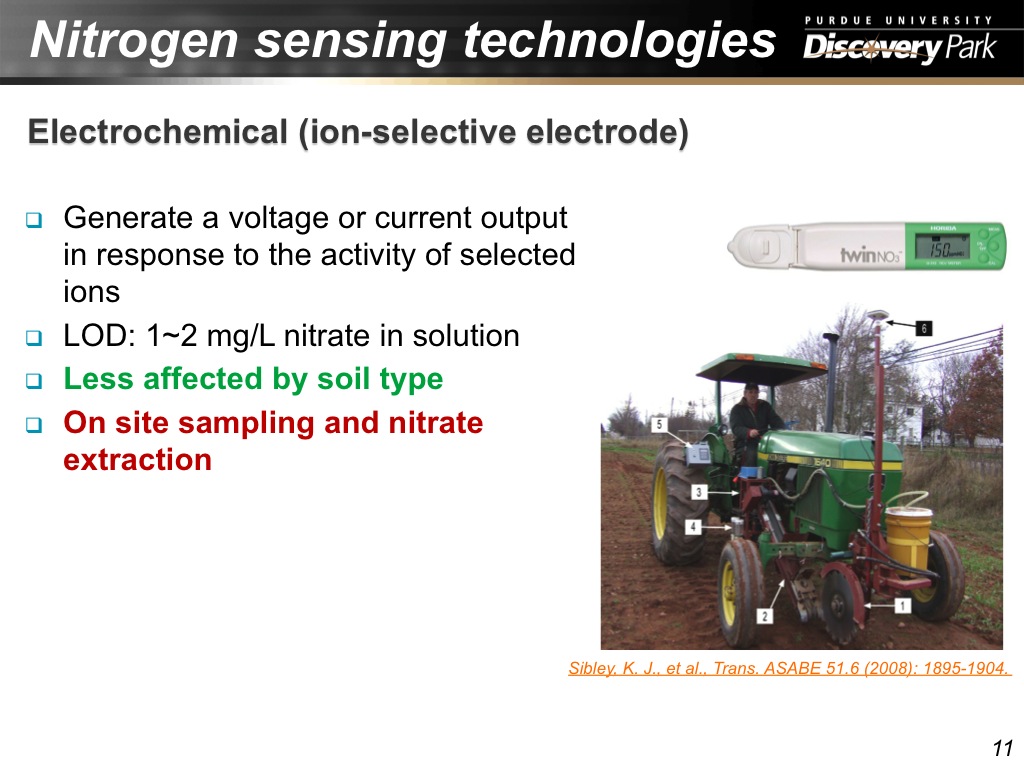 11. Nitrogen sensing technologies
539.27260593927258
00:00/00:00
11. Nitrogen sensing technologies
539.27260593927258
00:00/00:00 -
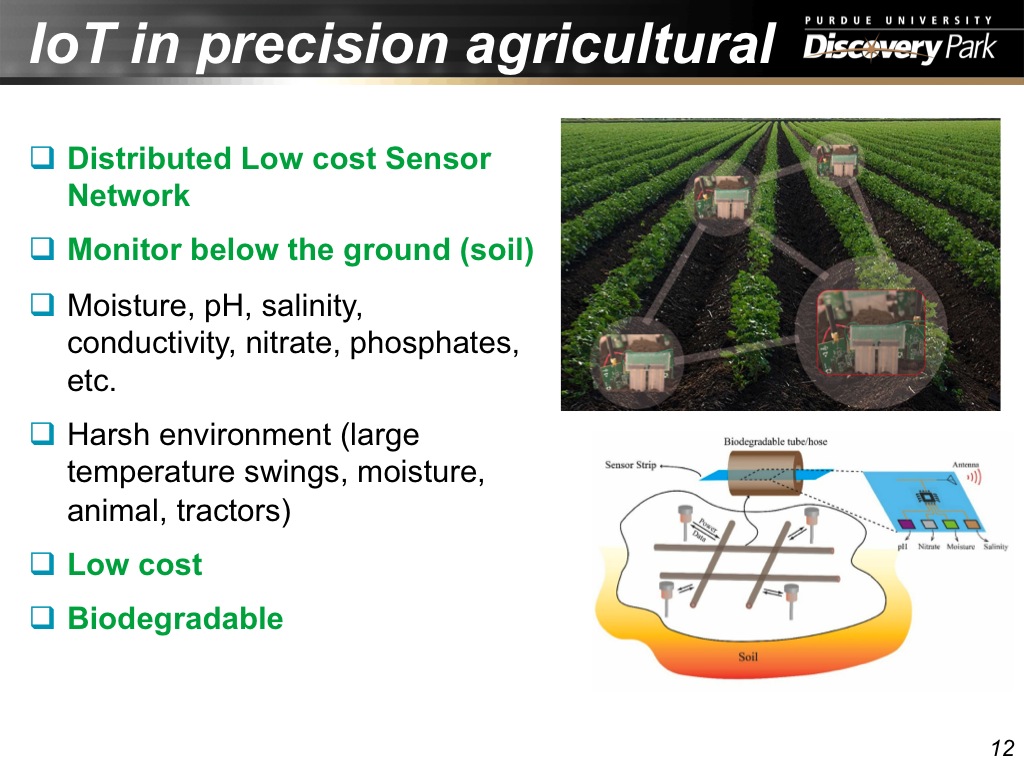 12. IoT in precision agricultural
587.28728728728731
00:00/00:00
12. IoT in precision agricultural
587.28728728728731
00:00/00:00 -
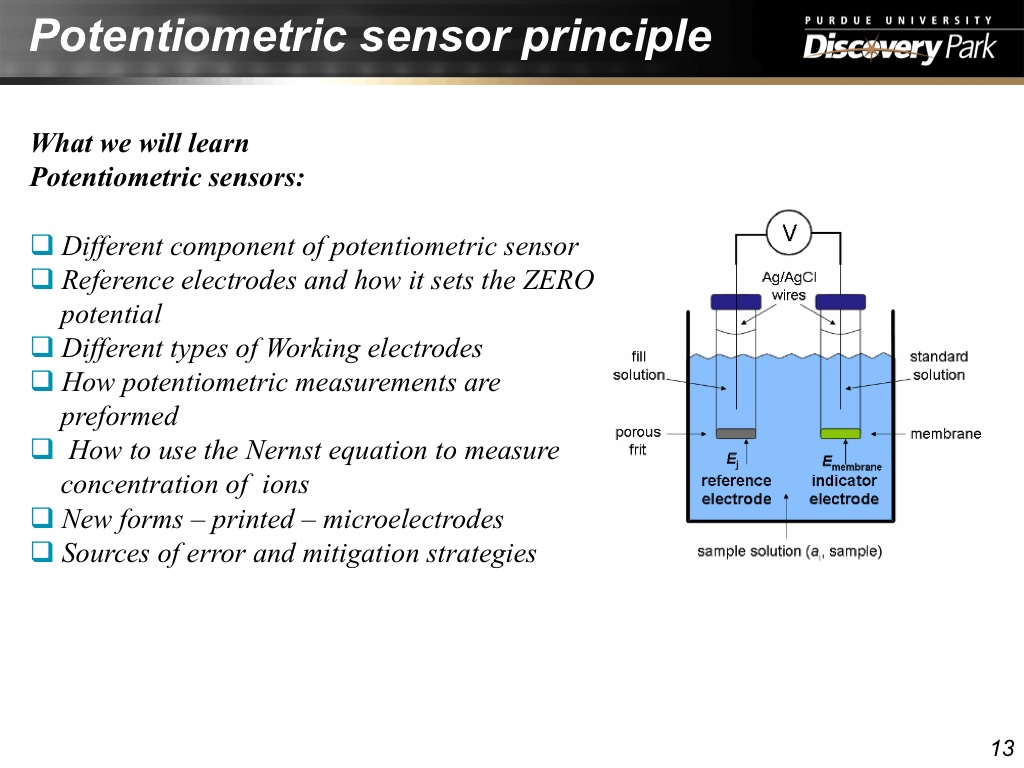 13. Potentiometric sensor principl…
643.44344344344347
00:00/00:00
13. Potentiometric sensor principl…
643.44344344344347
00:00/00:00 -
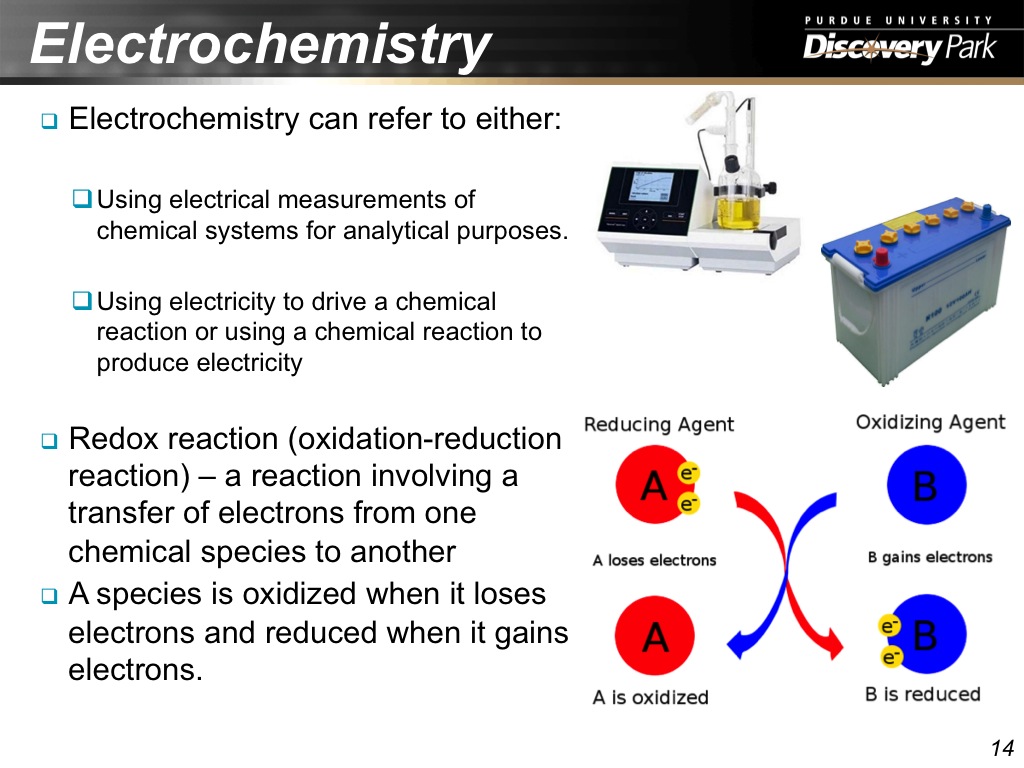 14. Electrochemistry
665.49883216549881
00:00/00:00
14. Electrochemistry
665.49883216549881
00:00/00:00 -
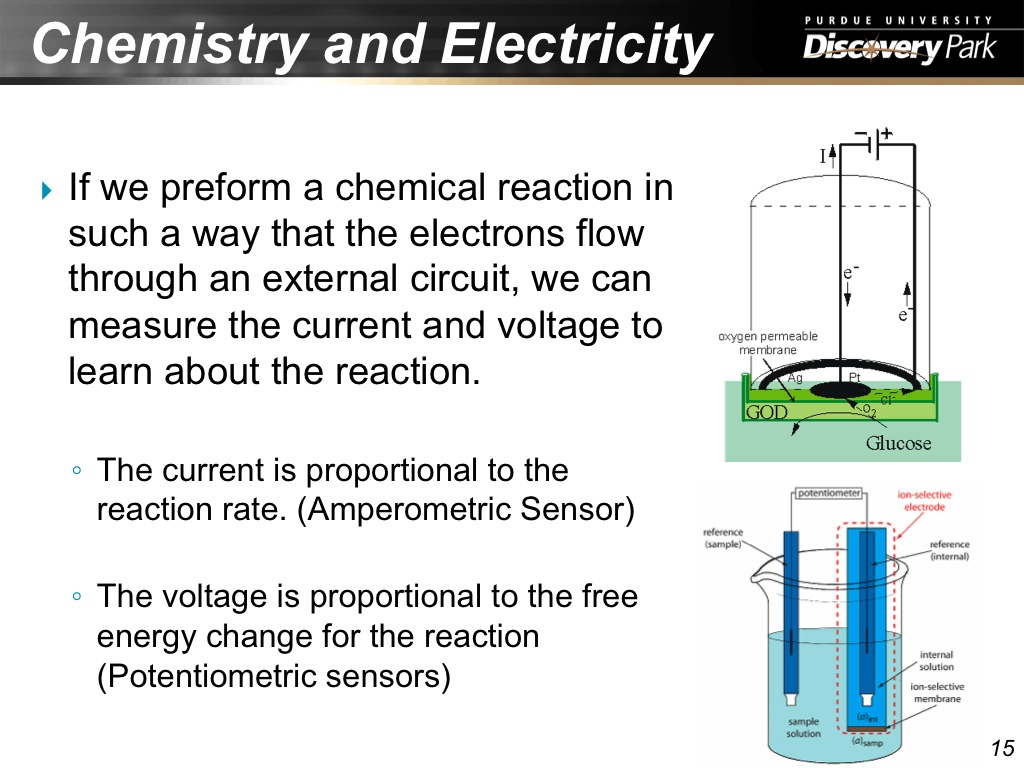 15. Chemistry and Electricity
727.69436102769441
00:00/00:00
15. Chemistry and Electricity
727.69436102769441
00:00/00:00 -
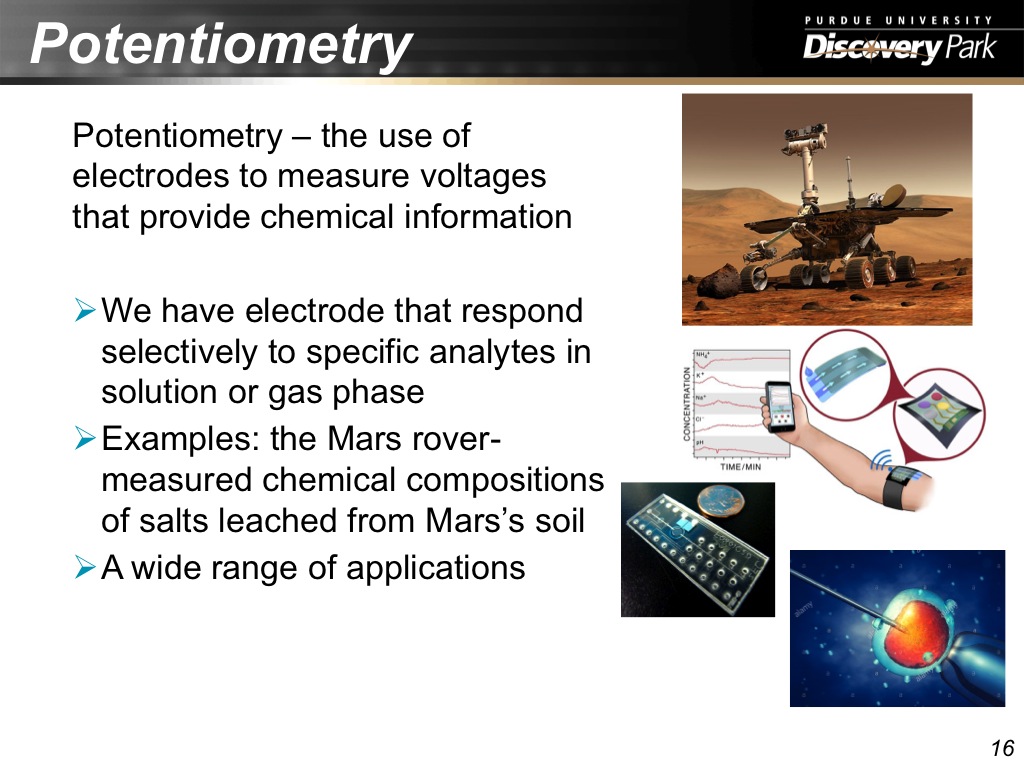 16. Potentiometry
764.56456456456465
00:00/00:00
16. Potentiometry
764.56456456456465
00:00/00:00 -
 17. Potential Energy
828.294961628295
00:00/00:00
17. Potential Energy
828.294961628295
00:00/00:00 -
 18. Potential Energy
870.37037037037044
00:00/00:00
18. Potential Energy
870.37037037037044
00:00/00:00 -
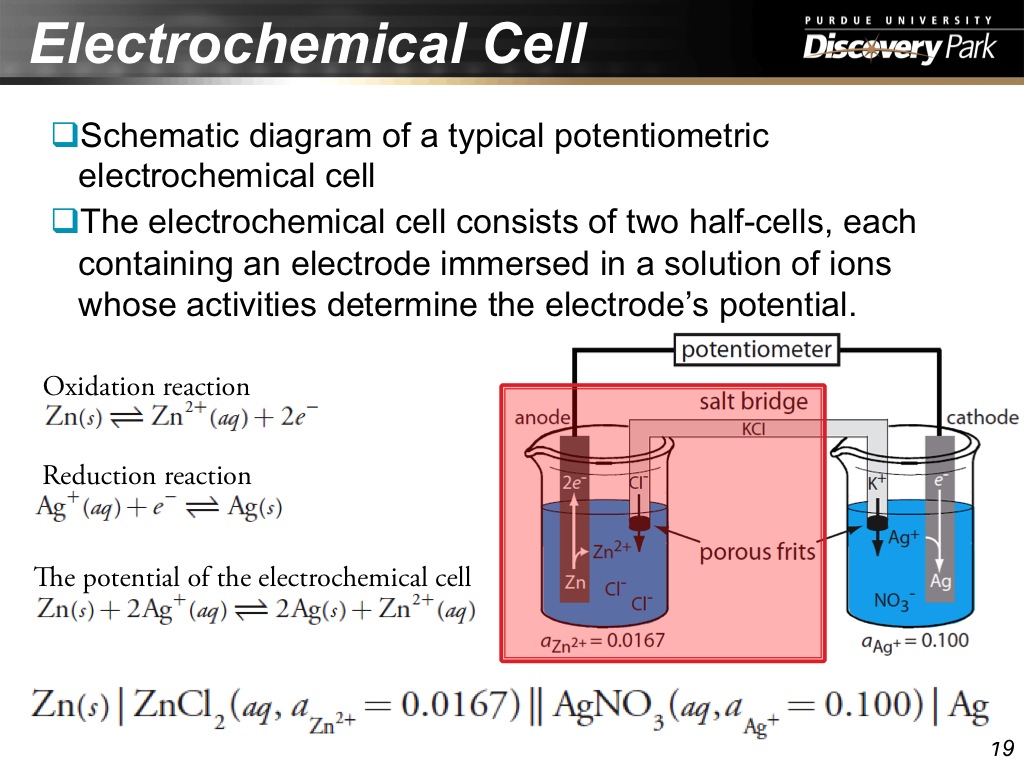 19. Electrochemical Cell
963.36336336336342
00:00/00:00
19. Electrochemical Cell
963.36336336336342
00:00/00:00 -
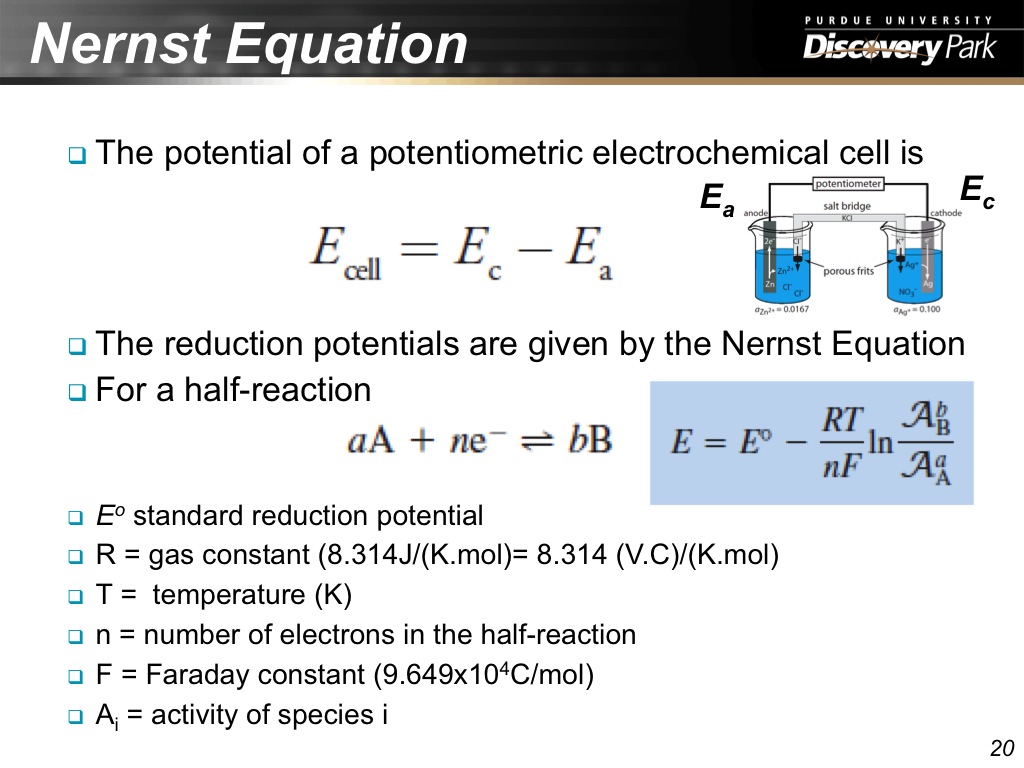 20. Nernst Equation
1047.3139806473141
00:00/00:00
20. Nernst Equation
1047.3139806473141
00:00/00:00 -
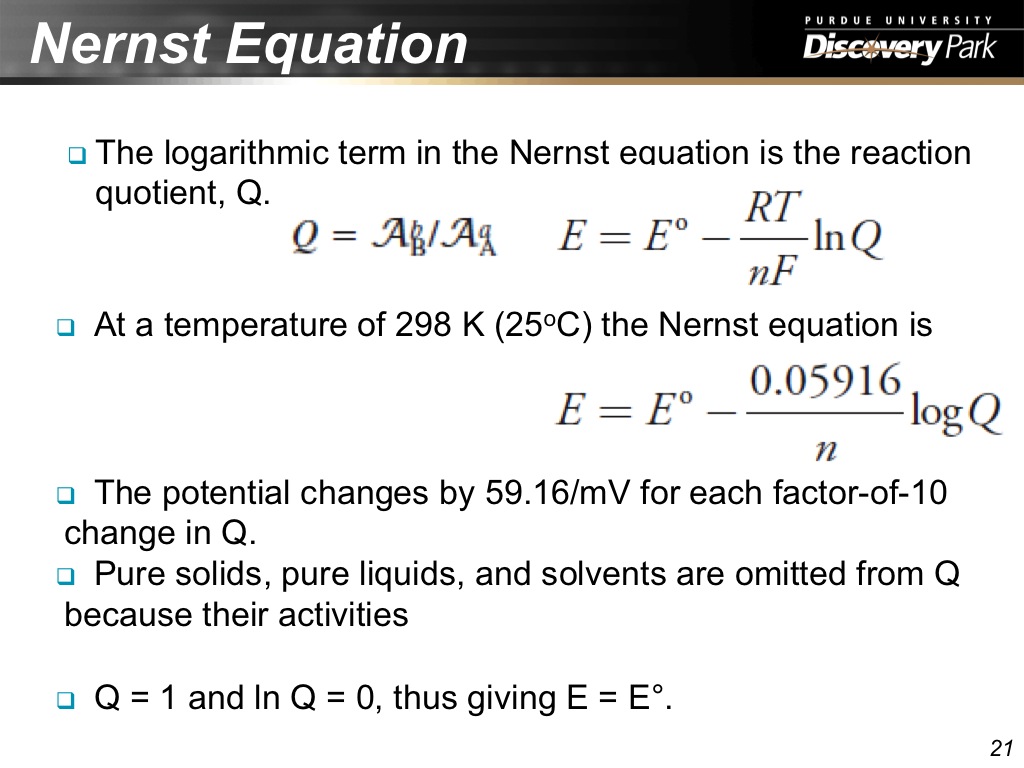 21. Nernst Equation
1093.3600266933602
00:00/00:00
21. Nernst Equation
1093.3600266933602
00:00/00:00 -
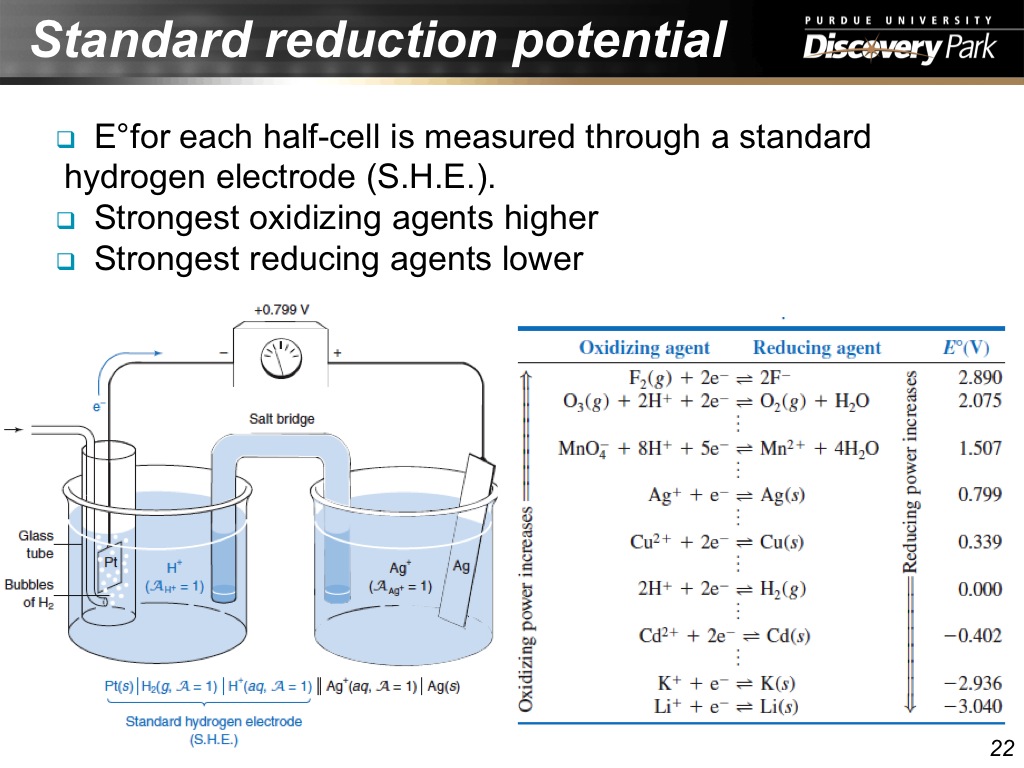 22. Standard reduction potential
1161.6282949616284
00:00/00:00
22. Standard reduction potential
1161.6282949616284
00:00/00:00 -
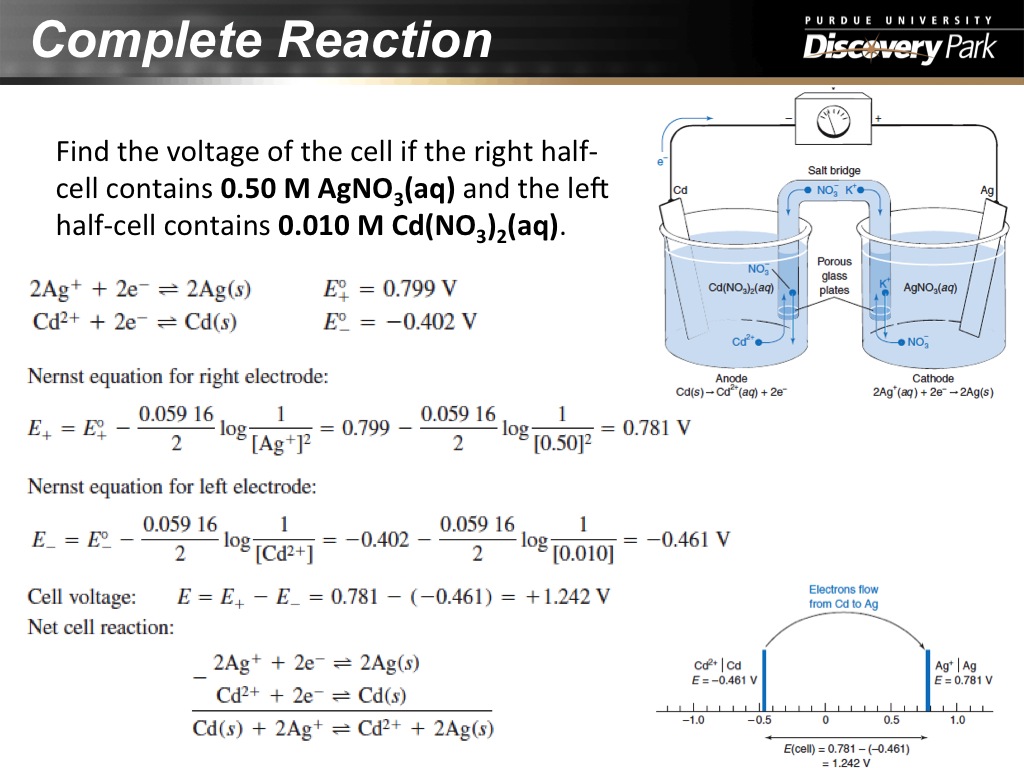 23. Complete Reaction
1197.097097097097
00:00/00:00
23. Complete Reaction
1197.097097097097
00:00/00:00 -
 24. Complete Reaction
1283.0163496830164
00:00/00:00
24. Complete Reaction
1283.0163496830164
00:00/00:00 -
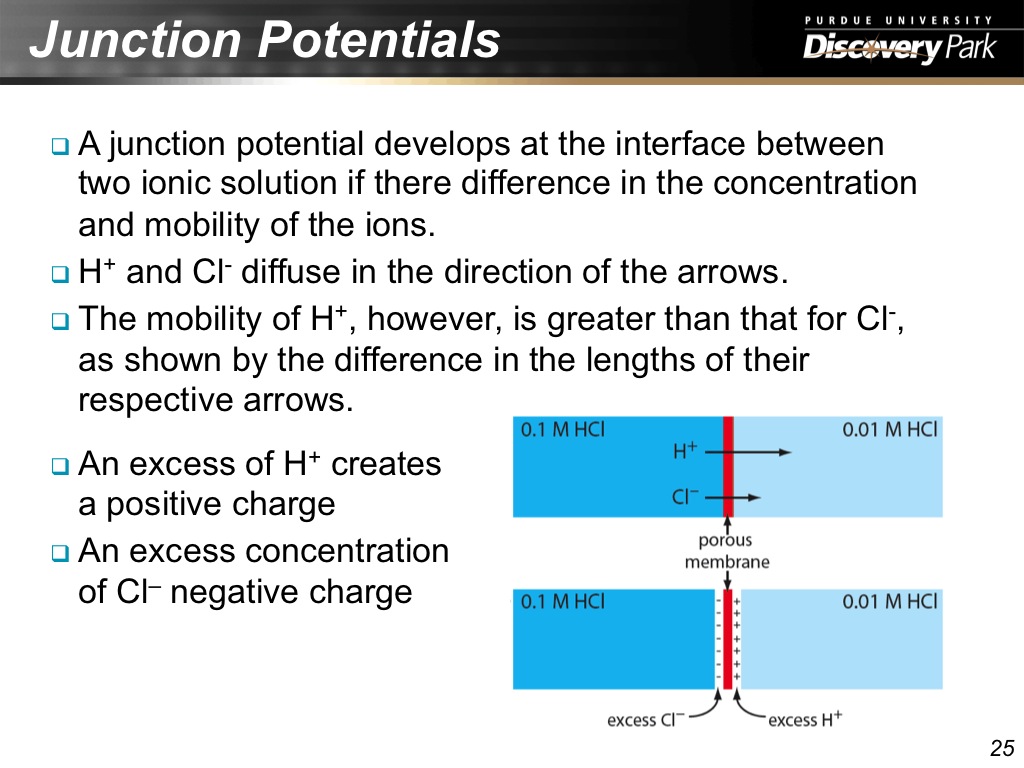
 25. Junction Potentials
1366.7000333667002
00:00/00:00
25. Junction Potentials
1366.7000333667002
00:00/00:00 -
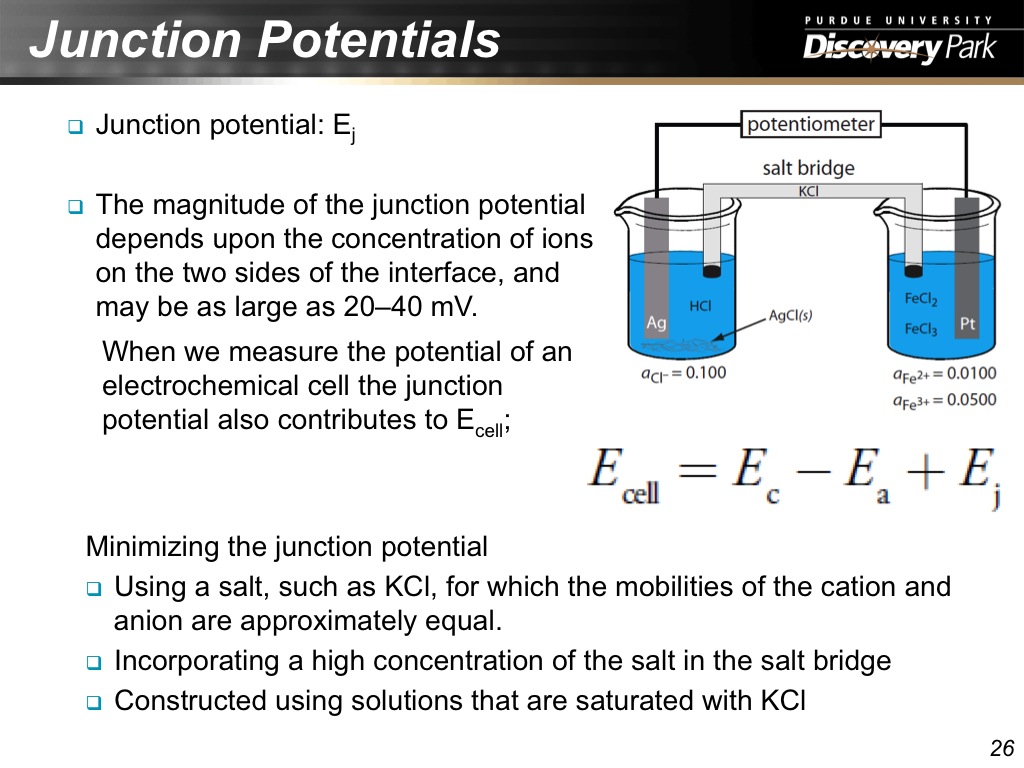
 26. Junction Potentials
1377.6776776776778
00:00/00:00
26. Junction Potentials
1377.6776776776778
00:00/00:00 -
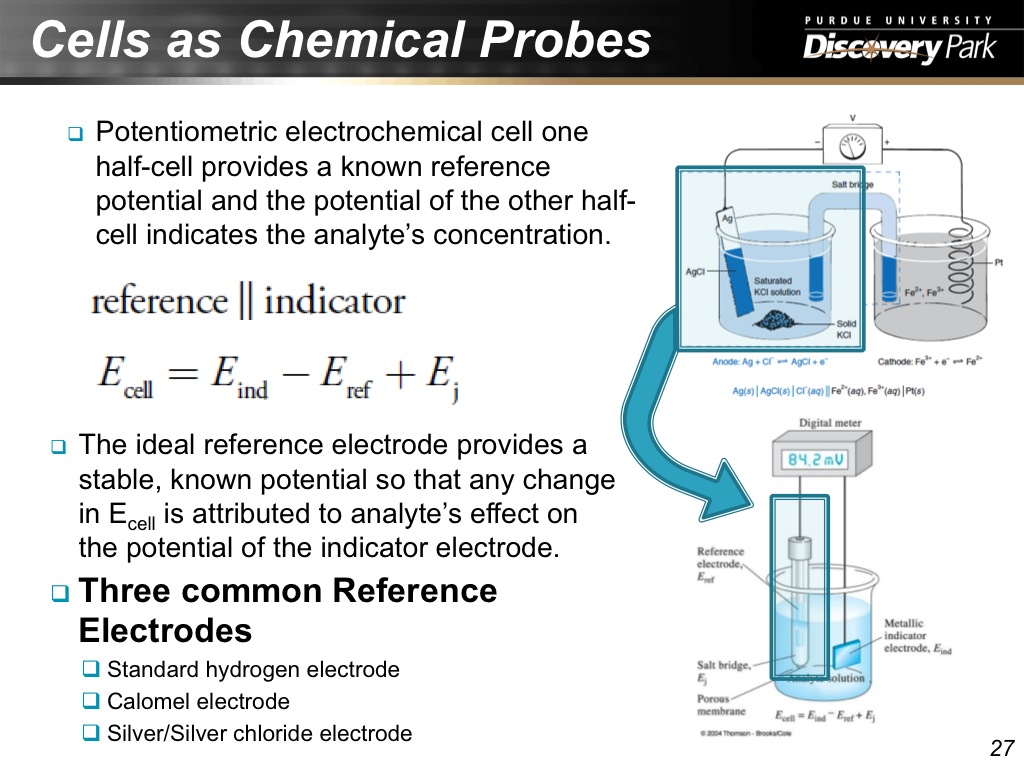
 27. Cells as Chemical Probes
1478.7787787787788
00:00/00:00
27. Cells as Chemical Probes
1478.7787787787788
00:00/00:00 -
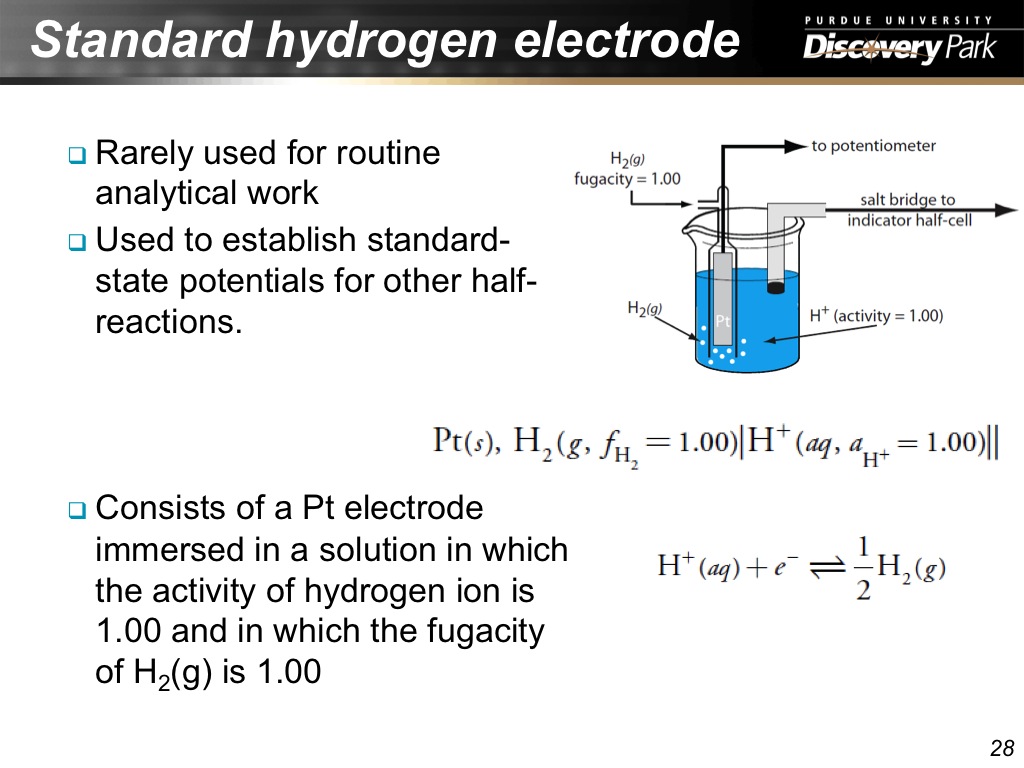
 28. Standard hydrogen electrode
1527.9946613279947
00:00/00:00
28. Standard hydrogen electrode
1527.9946613279947
00:00/00:00 -
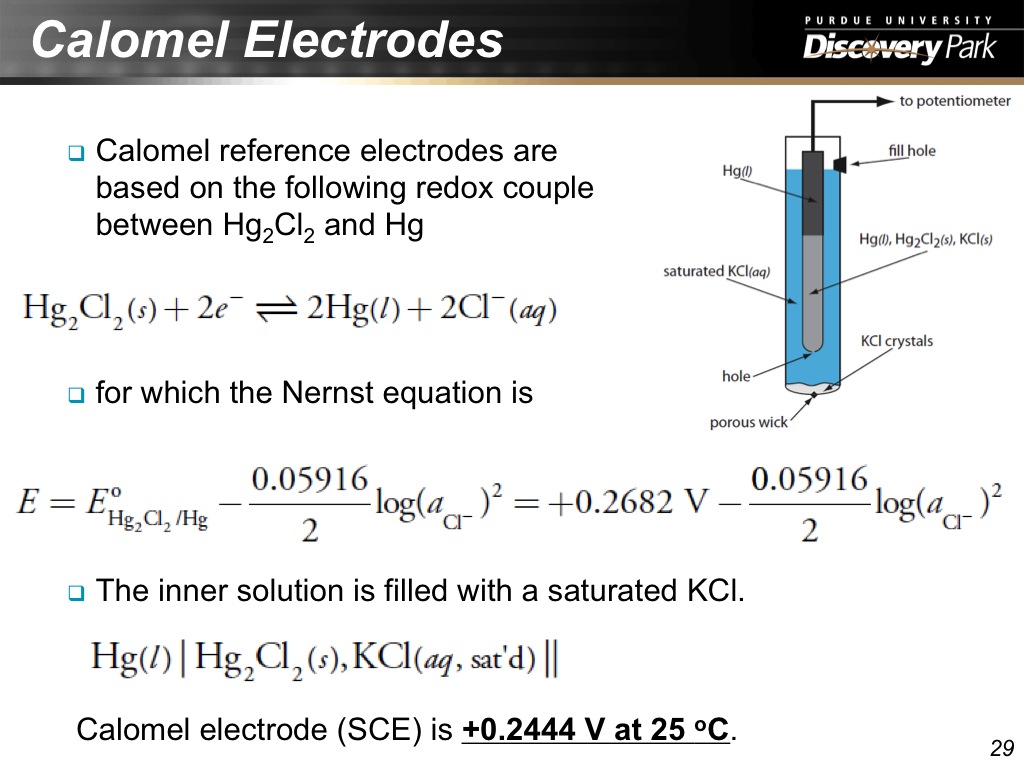
 29. Calomel Electrodes
1627.660994327661
00:00/00:00
29. Calomel Electrodes
1627.660994327661
00:00/00:00 -
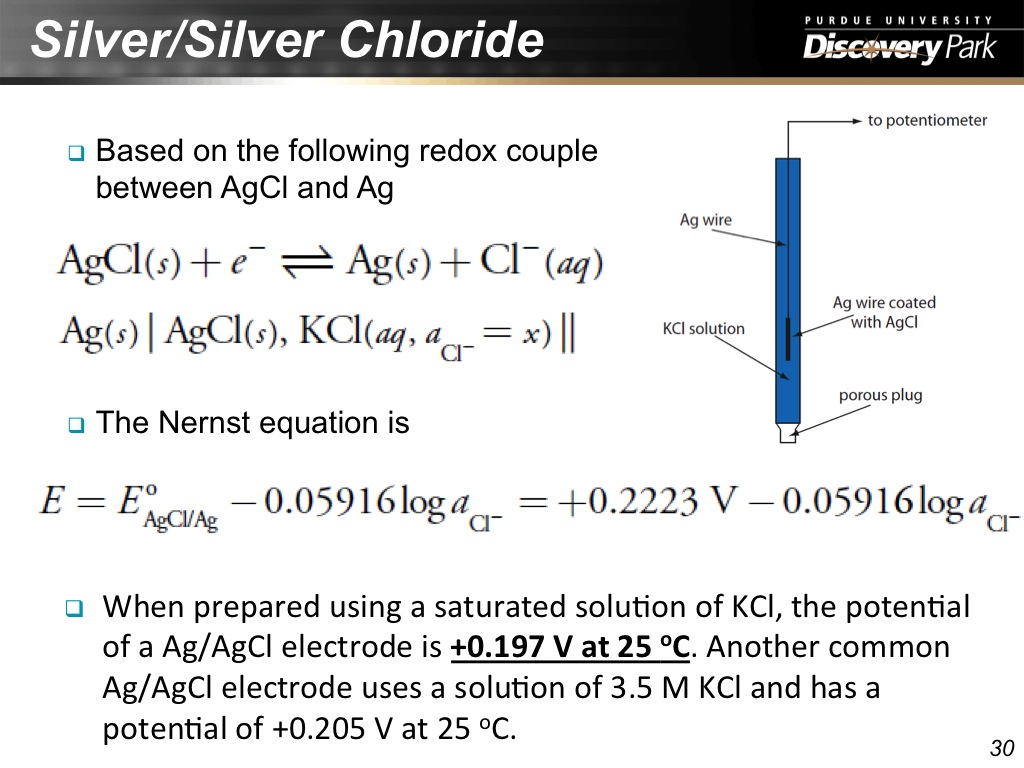
 30. Silver/Silver Chloride
1696.5632298965634
00:00/00:00
30. Silver/Silver Chloride
1696.5632298965634
00:00/00:00 -
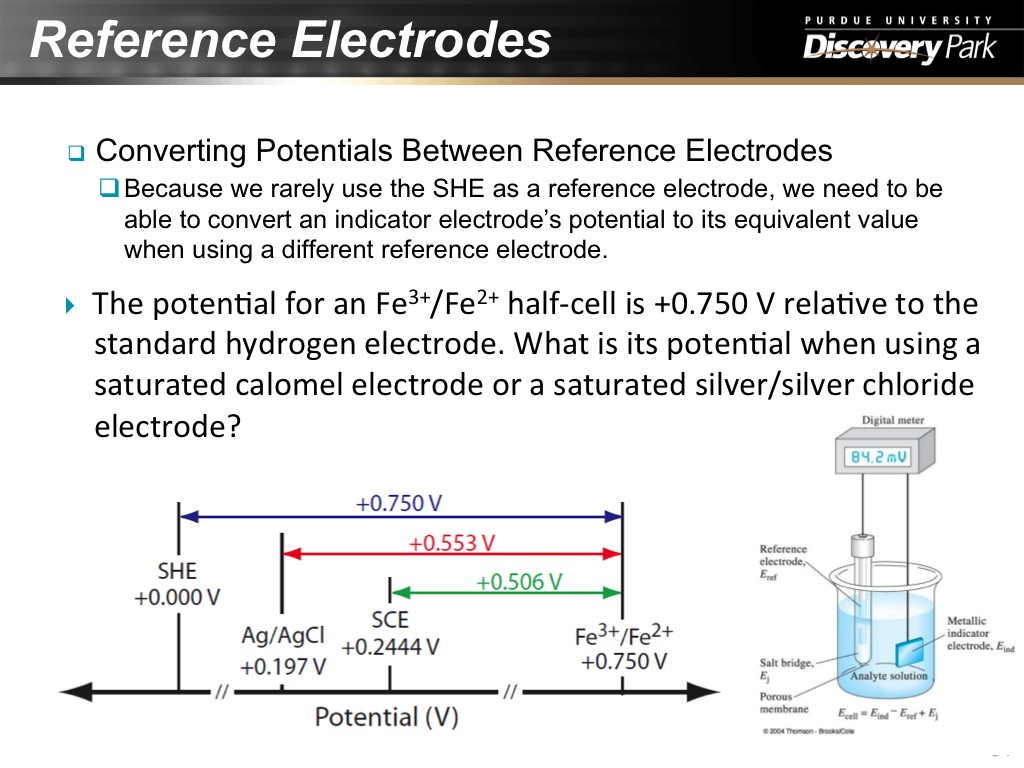
 31. Reference Electrodes
1752.9863196529864
00:00/00:00
31. Reference Electrodes
1752.9863196529864
00:00/00:00 -
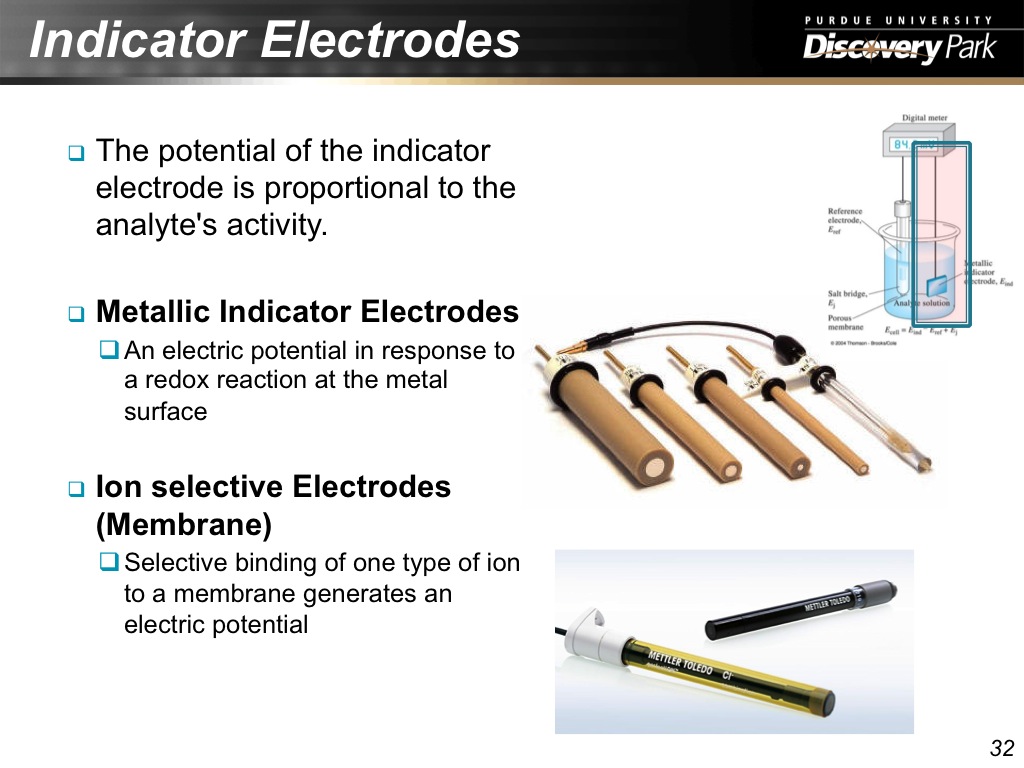
 32. Indicator Electrodes
1822.5558892225561
00:00/00:00
32. Indicator Electrodes
1822.5558892225561
00:00/00:00 -
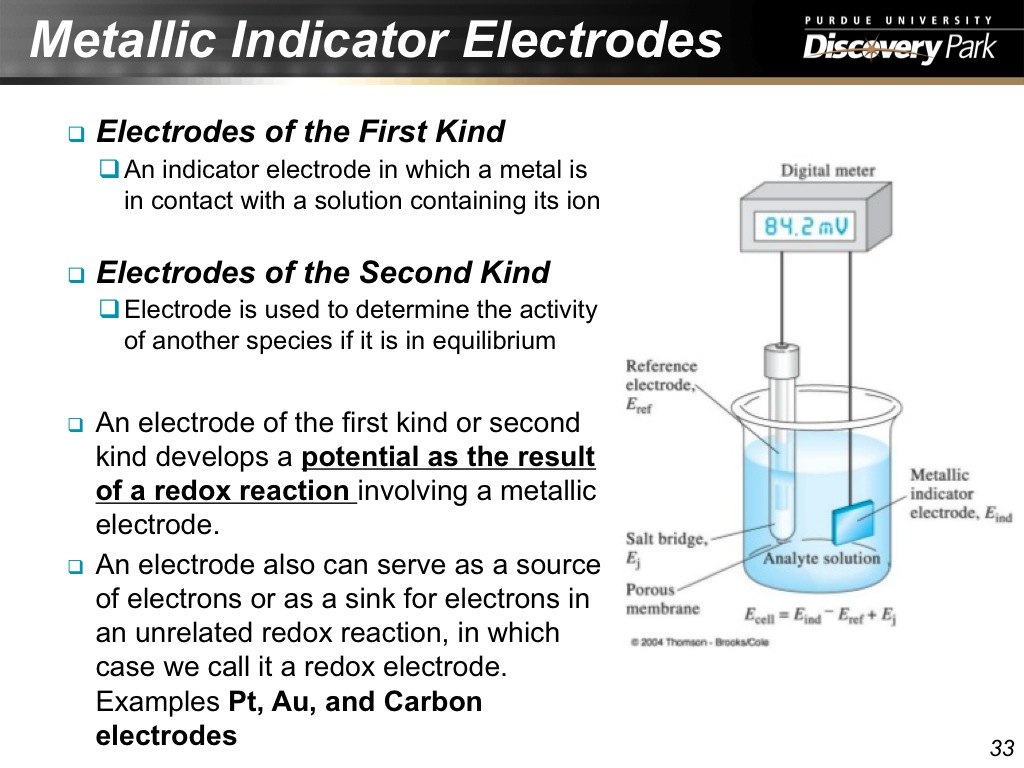
 33. Metallic Indicator Electrodes
1829.5962629295964
00:00/00:00
33. Metallic Indicator Electrodes
1829.5962629295964
00:00/00:00 -
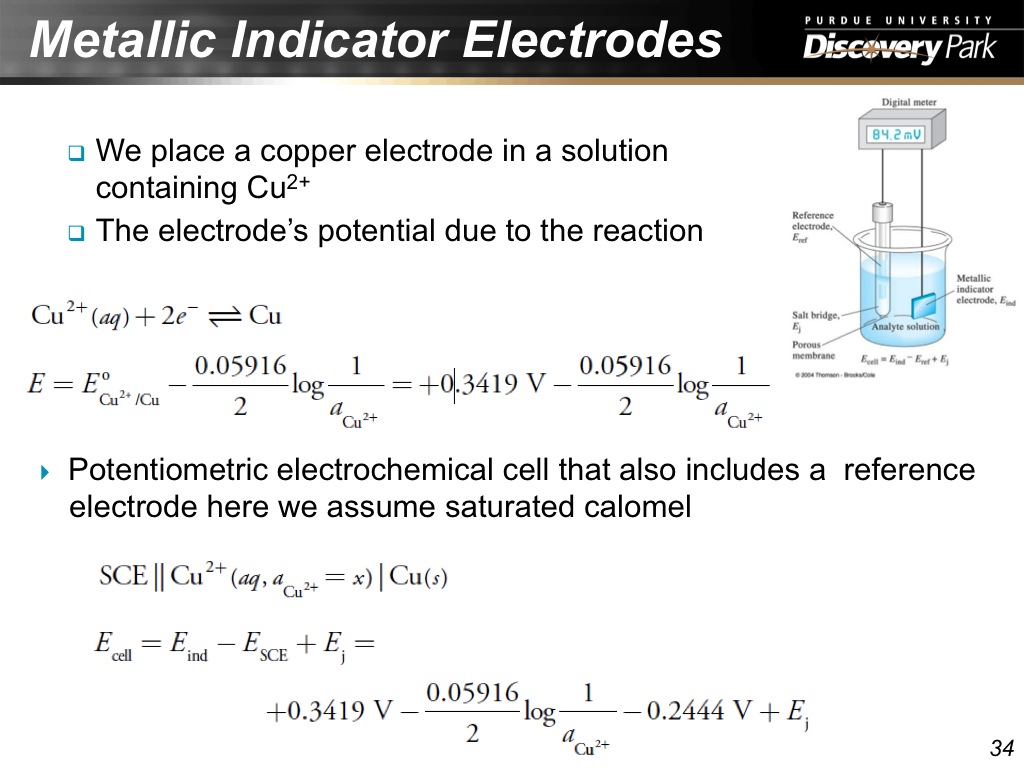
 34. Metallic Indicator Electrodes
1969.6029362696031
00:00/00:00
34. Metallic Indicator Electrodes
1969.6029362696031
00:00/00:00 -
 35. Membrane Electrodes
2012.6793460126794
00:00/00:00
35. Membrane Electrodes
2012.6793460126794
00:00/00:00 -
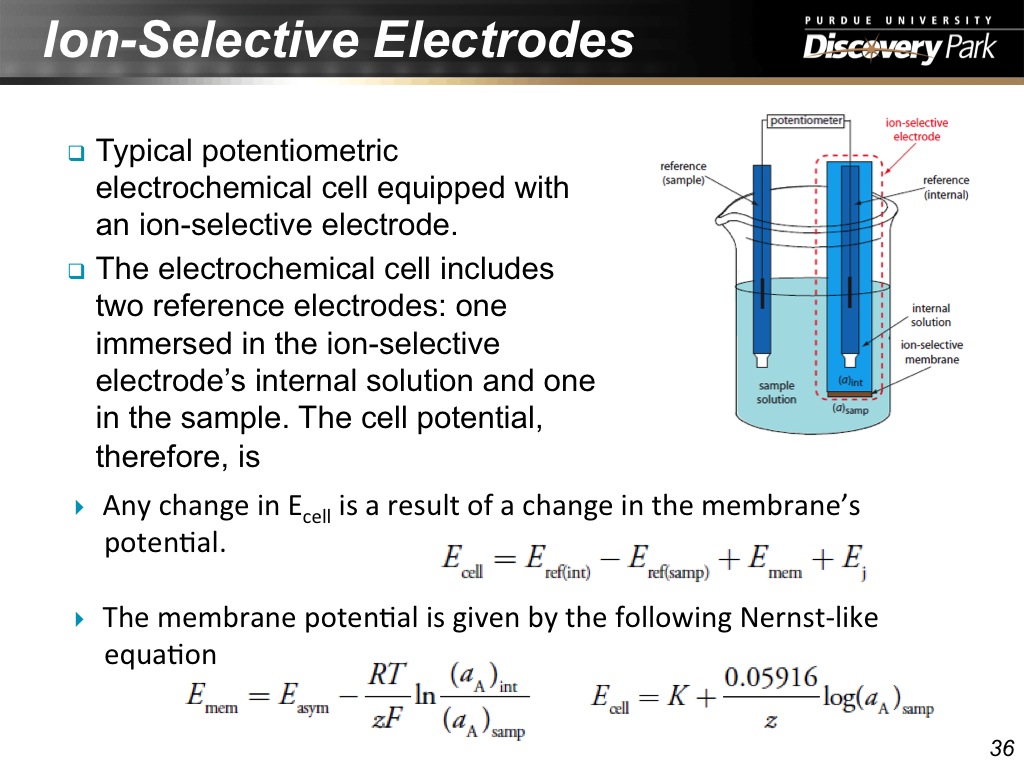
 36. Ion-Selective Electrodes
2142.2756089422755
00:00/00:00
36. Ion-Selective Electrodes
2142.2756089422755
00:00/00:00 -
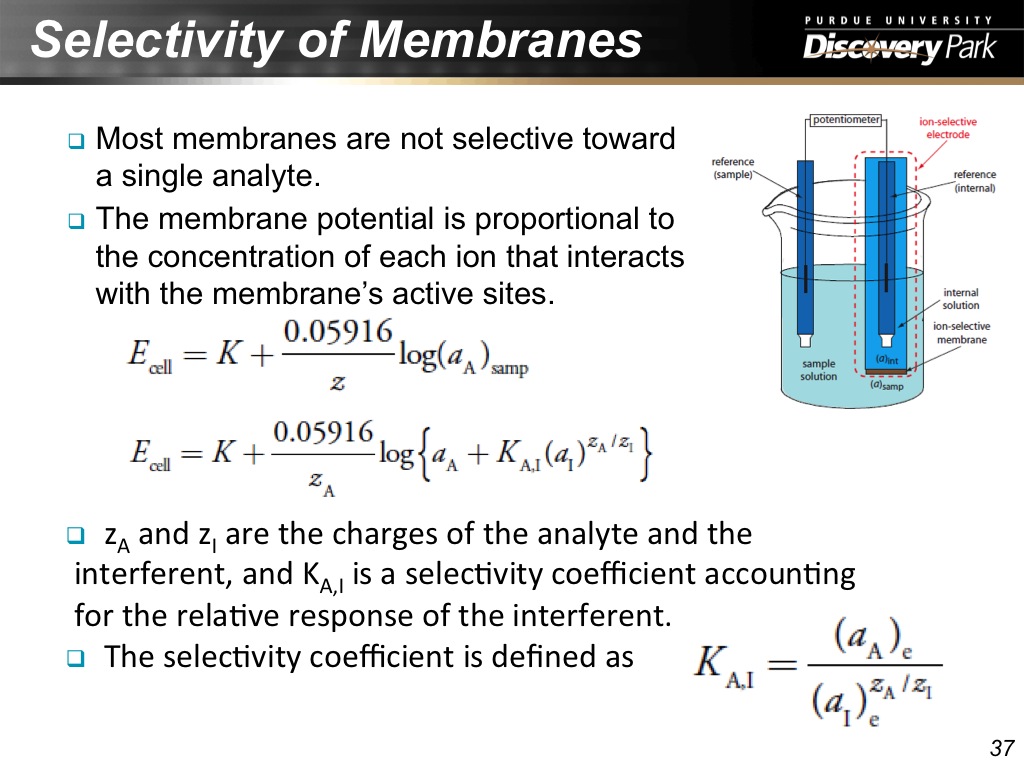
 37. Selectivity of Membranes
2185.9192525859194
00:00/00:00
37. Selectivity of Membranes
2185.9192525859194
00:00/00:00 -
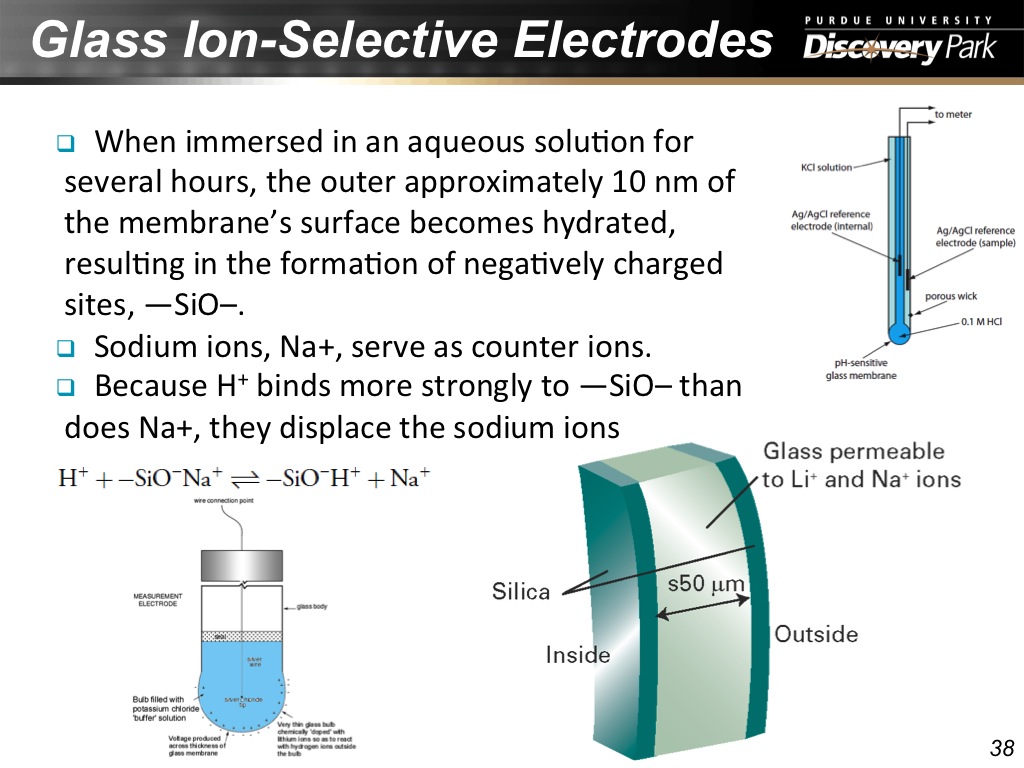
 38. Glass Ion-Selective Electrodes
2197.5975975975975
00:00/00:00
38. Glass Ion-Selective Electrodes
2197.5975975975975
00:00/00:00 -
 39. Glass pH electrode
2428.6619953286622
00:00/00:00
39. Glass pH electrode
2428.6619953286622
00:00/00:00 -
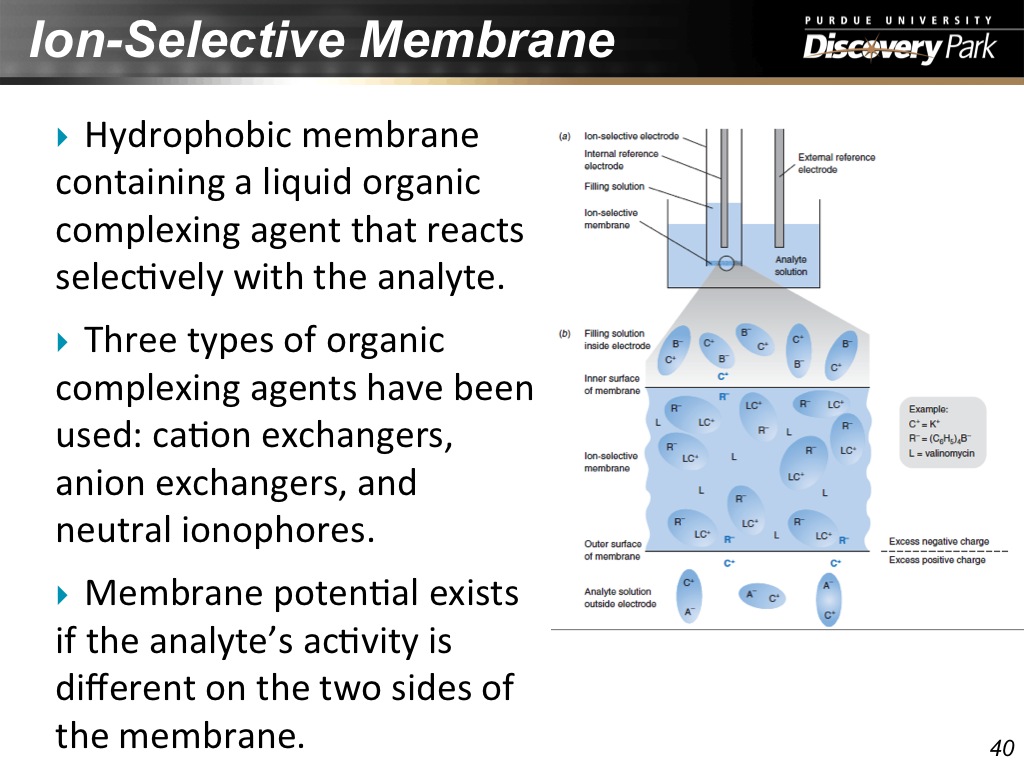
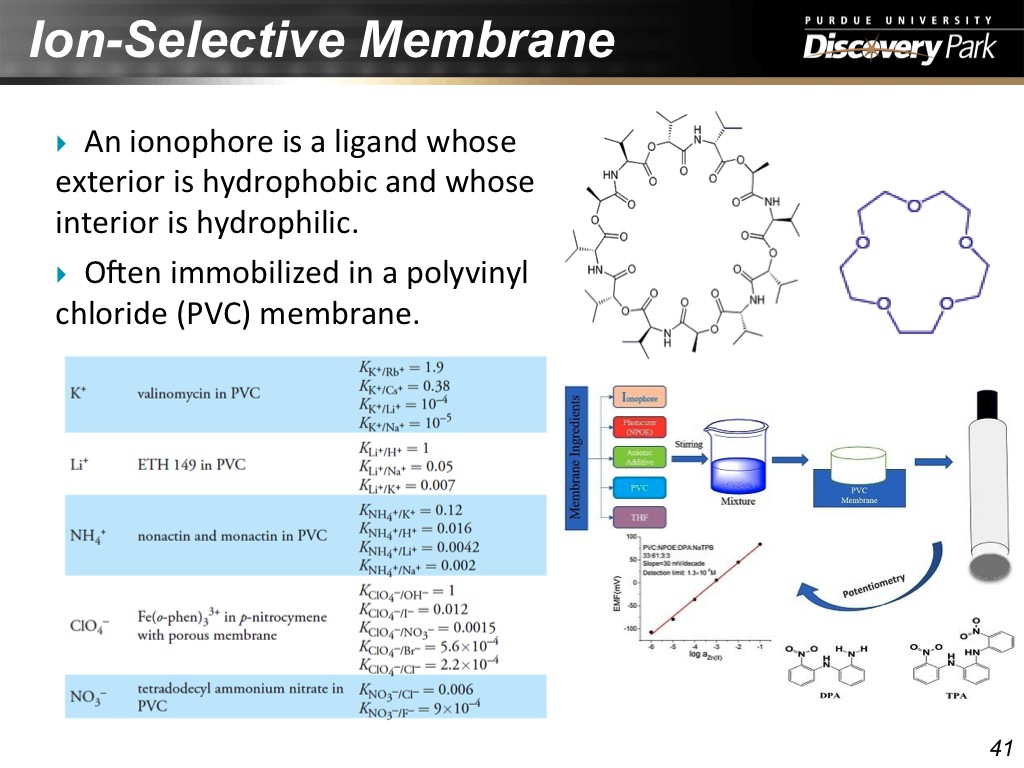 40. Ion-Selective Membrane
2460.3269936603269
00:00/00:00
40. Ion-Selective Membrane
2460.3269936603269
00:00/00:00 -
 41. Ion-Selective Membrane
2524.090757424091
00:00/00:00
41. Ion-Selective Membrane
2524.090757424091
00:00/00:00 -
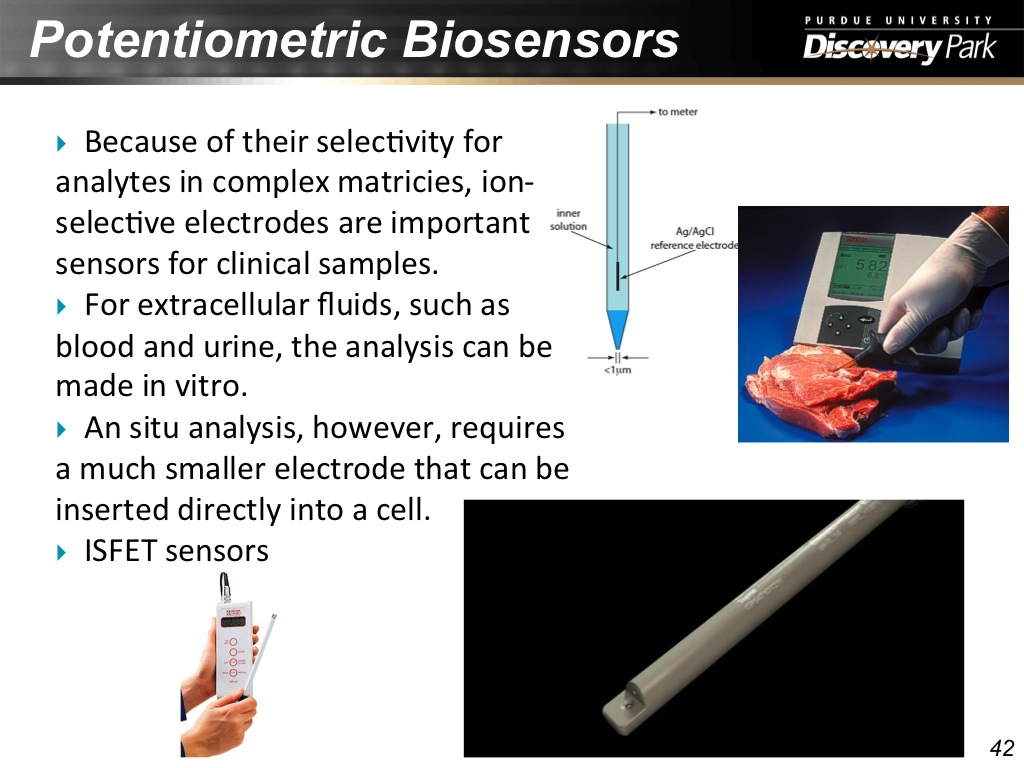
 42. Potentiometric Biosensors
2615.1484818151484
00:00/00:00
42. Potentiometric Biosensors
2615.1484818151484
00:00/00:00 -
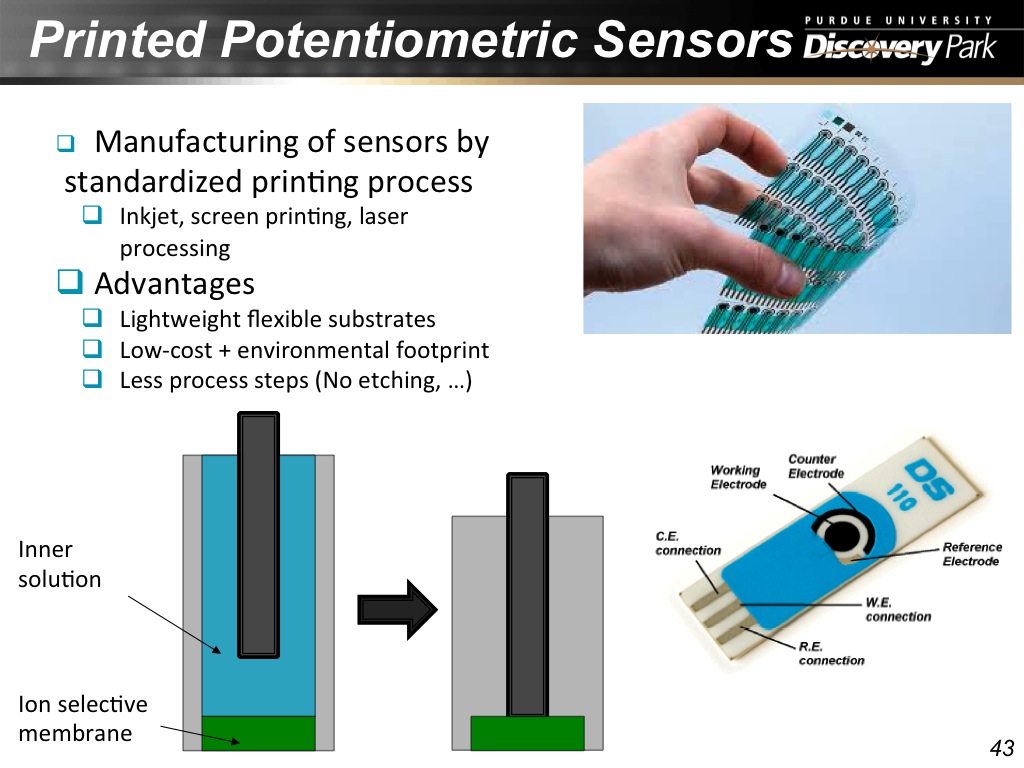
 43. Printed Potentiometric Sensors
2694.2275608942277
00:00/00:00
43. Printed Potentiometric Sensors
2694.2275608942277
00:00/00:00 -
 44. Reference electrode
2727.7944611277944
00:00/00:00
44. Reference electrode
2727.7944611277944
00:00/00:00 -
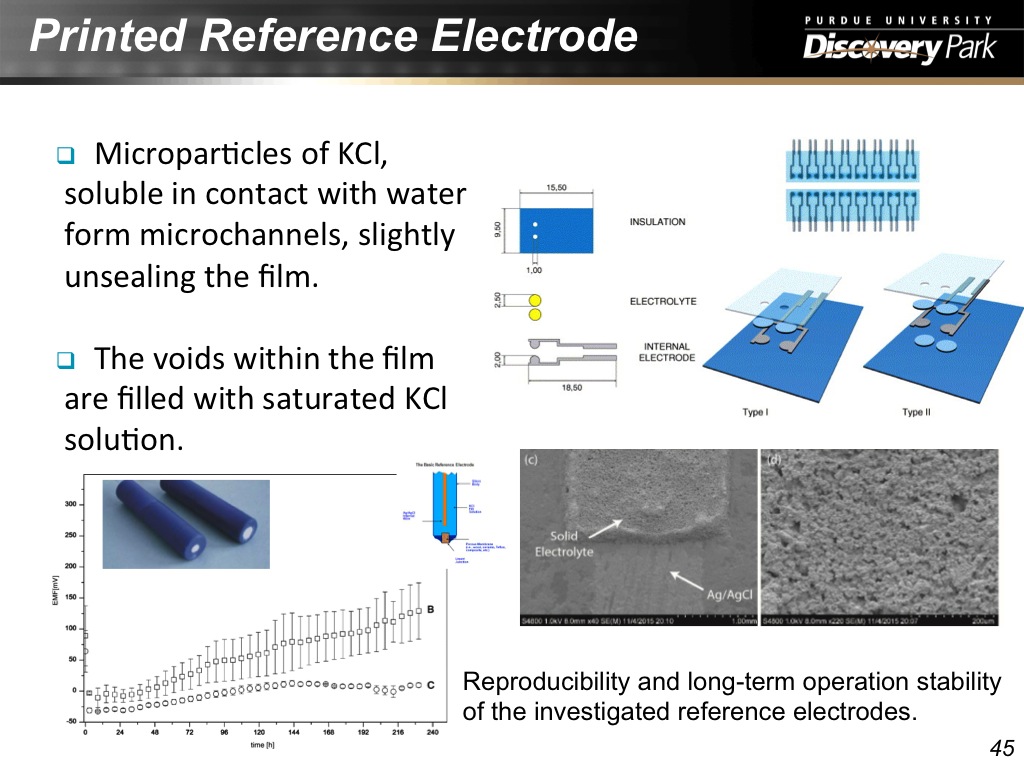
 45. Printed Reference Electrode
2813.1464798131465
00:00/00:00
45. Printed Reference Electrode
2813.1464798131465
00:00/00:00 -
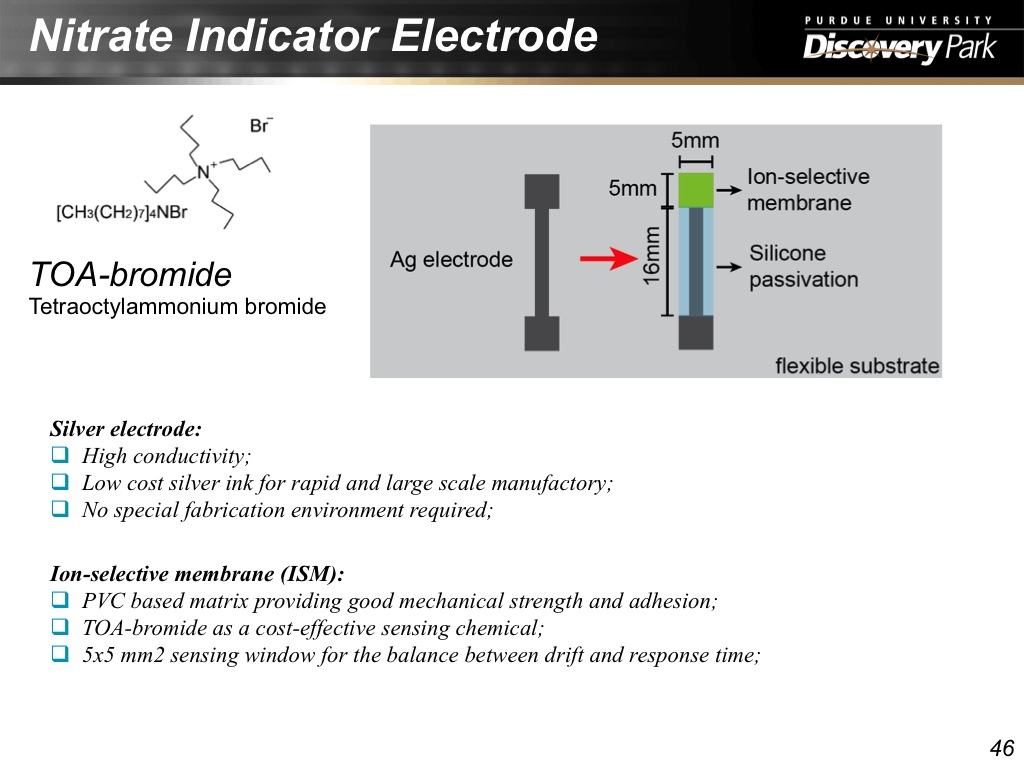
 46. Nitrate Indicator Electrode
2844.0106773440107
00:00/00:00
46. Nitrate Indicator Electrode
2844.0106773440107
00:00/00:00 -
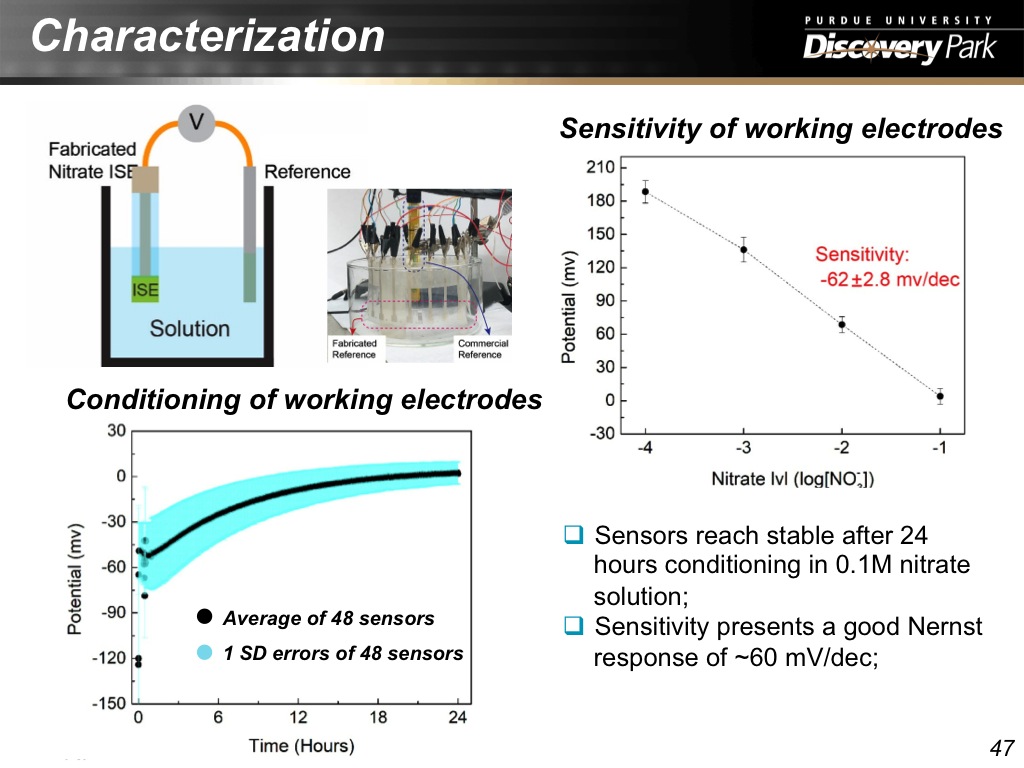
 47. Characterization
2909.1091091091093
00:00/00:00
47. Characterization
2909.1091091091093
00:00/00:00
 Rahim Rahimi is an Assistant Professor in the Materials Engineering department at Purdue University. He earned his B.S. (2008) and M.S. (2011) degrees in Electrical Engineering from the Iran University of Science and Technology, and his Ph.D. (2017) degree and post-doctoral (2018) in Electrical and Computer Engineering from the Purdue University, USA. His research has explored development of innovative, scalable, multifunctional, microsystem platforms for medical applications, with emphasis on smart wearable and autonomous devices for wound monitoring and therapy. His research on smart dressing for burn victims and stretchable embroidered electronics has been featured in various news media, including Science Nation, Science360, The Computer World, and Science X. During his graduate and post-graduate career, he has co-authored over 50 publications in world renowned journals and international conferences as well as book chapter and patents. Dr. Rahimi has also has led research teams on multi-institutional research endeavors focused on developing scalable manufacturing processes of flexible electronic devices that can empower technologies for health-care and environmental monitoring.
Rahim Rahimi is an Assistant Professor in the Materials Engineering department at Purdue University. He earned his B.S. (2008) and M.S. (2011) degrees in Electrical Engineering from the Iran University of Science and Technology, and his Ph.D. (2017) degree and post-doctoral (2018) in Electrical and Computer Engineering from the Purdue University, USA. His research has explored development of innovative, scalable, multifunctional, microsystem platforms for medical applications, with emphasis on smart wearable and autonomous devices for wound monitoring and therapy. His research on smart dressing for burn victims and stretchable embroidered electronics has been featured in various news media, including Science Nation, Science360, The Computer World, and Science X. During his graduate and post-graduate career, he has co-authored over 50 publications in world renowned journals and international conferences as well as book chapter and patents. Dr. Rahimi has also has led research teams on multi-institutional research endeavors focused on developing scalable manufacturing processes of flexible electronic devices that can empower technologies for health-care and environmental monitoring.